The Nexian DMT Handbook
| Note: | This page is a work in progress -- its content throughout is not yet complete. |
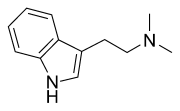
The production and use of DMT (N,N-Dimethyltryptamine), otherwise known as "Spice", is a practice that resonates strongly with the complementary qualities of ancient shamanic and alchemical spiritual practice as well as contemporary DIY (Do It Yourself) ethic. The production of spice is a discipline unlike that of most other commonly manufactured drugs, as it is not as well suited for bulk-production nor production for the purpose of sale as most well-known and intensively manufactured substances. As such, its use is generally inseparable from its production in practice and in spirit.
The production of DMT most commonly entails its extraction from botanical sources and only very rarely entails its synthesis. In this way, its production still strongly resembles its more ancient preparations by manner of brewing, a simple form of aqueous extraction still commonly performed to this day. This is the simplest and most readily administered form of extract if used as a component of a harmaloid-based preparation or -huasca brew.
Please take the time to seek further elaboration at the following resources:
Contents
- 1 Source Selection
- 2 General Plant Info
- 3 Identification
- 4 Alkaloid content
- 5 Extraction teks
- 6 Cultivation
- 7 Refinery for the Purpose of Extraction
- 8 References
- 9 Links
- 10 Extraction
- 11 Crystallization
- 12 The FASA Method
- 13 Purification
- 14 Administration
- 15 Appendices
- 16 Brief overview - What is DMT N-Oxide?
- 17 Chemical and physical properties
- 18 Effects
- 19 Pharmacology, toxicity and general safety
- 20 Plants containing DMT N-Oxide
- 21 Extraction teks
- 22 Dosages and consumption methods
- 23 History of usage
- 24 Analysis of DMT N-Oxide
- 25 Scientific publications
- 26 Other links of interest
- 27 Brief overview - What is 5-MeO-DMT?
- 28 Chemical and physical properties
- 29 Effects
- 30 Pharmacology, toxicity and general safety
- 31 Life-forms containing 5-MeO-DMT
- 31.1 Acacia spp.
- 31.2 Anadenanthera spp.
- 31.3 Arundo spp.
- 31.4 Bromus spp.
- 31.5 Caesalpinia spp.
- 31.6 Delosperma spp.
- 31.7 Desmodium spp.
- 31.8 Dictyoloma spp.
- 31.9 Dutaillyea spp.
- 31.10 Digitaria spp.
- 31.11 Diplopterys spp.
- 31.12 Evodia spp.
- 31.13 Horsfieldiana spp.
- 31.14 Hugonia spp.
- 31.15 Iryanthera spp.
- 31.16 Justicia spp.
- 31.17 Lespedeza spp.
- 31.18 Mimosa spp.
- 31.19 Mucuna spp.
- 31.20 Osteophloem spp.
- 31.21 Other Grasses
- 31.22 Phalaris spp.
- 31.23 Sorghum spp.
- 31.24 Umbellularia spp.
- 31.25 Virola spp.
- 31.26 Humans and other animals
- 32 Extraction teks
- 33 Dosages and consumption methods
- 34 History of usage
- 35 Analysis of 5-MeO-DMT
- 36 Scientific publications
- 37 Other links of interest
- 38 Brief overview - What is Bufotenine?
- 39 Chemical and physical properties
- 40 Effects
- 41 Pharmacology, toxicity and general safety
- 42 Life forms containing bufotenine
- 42.1 Amanita spp.
- 42.2 Anadenanthera spp.
- 42.3 Arundo spp.
- 42.4 Desmodium spp.
- 42.5 Lespedeza spp.
- 42.6 Mimosa spp.
- 42.7 Mucuna spp.
- 42.8 Osteophloem spp.
- 42.9 Piptadenia spp.
- 42.10 Phalaris spp.
- 42.11 Phragmites spp.
- 42.12 Umbellularia spp.
- 42.13 Urtica spp.
- 42.14 Virola spp.
- 42.15 Humans and other animals
- 43 Extraction teks
- 44 Dosages and consumption methods
- 45 History of usage
- 46 Analysis of bufotenine
- 47 Scientific publications
- 48 Other links of interest
Source Selection
DMT, its analogues, and other related alkaloids can be found in a wide variety of lifeforms, varying from trace amounts to considerable amounts. It is impossible, therefore, to include all of the sources from which DMT can be extracted, so the following discussion will focus primarily on the most commonly used and significant botanical sources.
Botanical Considerations
Several species of plants contain a variety of constituents apart from DMT. This consideration is of the utmost importance when selecting the source plant from which an extraction is to be performed, as it may become the determining factor in the material requirements of the extraction process. Some plants may even contain toxic alkaloids, so thorough research must be conducted prior to selection, extraction, and administration.
Common Botanical Sources
General Plant Info
Mimosa hostilis is the former scientific name for Mimosa tenuiflora, and the two names are synonymous [1][2]. The older name is still widely know due to its presence in the literature and as distributers of botanical products still use the older term. M. tenuiflora is an entheogen known as Jurema, Jurema Preta, Black Jurema, and Vinho de Jurema. Dried Mexican Mimosa Hostilis root bark has been recently shown to have a DMT content of about 1%. The stem bark has about 0.03% DMT.
To date no β-carbolines such as harmala alkaloids have been detected in Mimosa tenuiflora decoctions, however the isolation of a new compound called "Yuremamine" from Mimosa tenuiflora as reported in 2005 represents a new class of phyto-indoles [3]. This may explain the reported oral activity of DMT in Jurema without the addition of an MAOI. Imported MHRB typically requires the addition of an MAOI in the preparation of ayahuasca.
Identification
Alkaloid content
- Root Bark contains DMT - 0.31% to 0.57% (Schultes 1977)
- Inner root bark contains up to 2% active alkaloids (Extractions from DMT-Nexus and others)
- 3% of the total alkaloids (or 0.04% of rootbark) is NMT and 2-Methyl-1,2,3,4-Tetrahydro-Beta-Carboline (Analysis of jungle spice, Analysis of red/yellow/white spices
Extraction teks
For extracting DMT , any of the extraction teks described here will work.
Yuremamine is sensitive to heat and pH changes so only cold water (or alcoholic) soak will retrieve it.
Cultivation
Growing: Mimosas aren´t cold proof. For outdoor growing they deserve a sunny place with leachy middle nutrient soil. Throughout the vegetation are copiously watered, in winter the watering is tied down on to the minimum. They are breeding with the seeds, but can be breeded with the cutting also.
Refinery for the Purpose of Extraction
Mimosa Hostilis Root Bark can be acquired in different stages of preparation. Usually it is sold as whole, shredded or pre-powdered root-bark, but one may have access to the whole root—usually when harvested directly.
- The whole root must cleaned and stripped of its inner root-bark while discarding the rest of the root.
- The whole root-bark must generally be torn by hand, cut, or smashed with a blunt object prior to shredding.
- The shredded should be further broken down as much as possible by peeling/cutting/blending to increase surface area for alkaloids to be extracted.
- The pre-powdered can always be used "as-is".
Below details how to break it from whole root
The Root
| Note: | Only the Inner Root Bark is necessary for extraction, the core and outer parts are to be discarded! |
| Root preparation | ||||
|---|---|---|---|---|
|
Pictured below is its original after being harvested from the plant. Notice the middle core is quite distinct from the root-bark, the outer bark is much more brown: |
Cleaning the root
Peeling the Inner Root Bark
| Peeling the Inner Root Bark | |||||
|---|---|---|---|---|---|
|
Once the outermost part has been removed, peel off the Inner Root Bark to separate it from the core. This can easily be accomplished immediately by hand, though the use of a knife may be helpful. Here's the inner core which is to be discarded: |
Result of root preparation
| Result of root preparation | ||
|---|---|---|
|
The peeled inner root-bark now needs to dry. This may be accomplished by simply leaving it in the sun. Here's how it should look: |
Breaking the rootbark up
| Breaking the rootbark up | |
|---|---|
|
The pieces/strips of inner root-bark require further refinery to expose a larger surface area and increase the availability of the alkaloids for extraction. If storage is desired, then the whole pieces are preferable, as the alkaloids are less exposed and thus better protected. First strip the pieces further into thinner layers with the hands, then cut it up with good scissors into smaller squares, then break it down in small amounts and short/medium bursts with a blender or coffee grinder (to prevent breaking of blender/grinder) |
References
- ↑ USDA, ARS, National Genetic Resources Program. Germplasm Resources Information Network - (GRIN) [Online Database]. National Germplasm Resources Laboratory, Beltsville, Maryland. URL: http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?24430
- ↑ Lewis, G.P. (1987) Royal Botanic Gardens, Kew 369 pp Legumes of Bahia.
- ↑ Vepsäläinen, Jouko J.; Auriola, Seppo; Tukiainen, Mikko; Ropponen, Nina & Callaway, J.C. (2005). "Isolation and characterization of yuremamine, a new phytoindole". Planta Medica, 71: 1053-1057. URL: http://www.ncbi.nlm.nih.gov/pubmed/16320208
Links
| Diplopterys cabrerana (Chaliponga
) |
|
|---|---|
|
| Psychotria viridis (Chacruna
) |
|
|---|---|
|
See also:
Methods of Refinery
Before extracting alkaloids from the variety of plants named above, one generally needs to clean and prepare the plant source, to include washing, pulverization, or any other necessary pre-treatment. Oftentimes, plants obtained through vendors have been prepared to some extent, and only require some degree of pulverization unless obtained in pre-powdered form. Pulverization is generally achieved by use of a household coffee grinder or a blender, though some materials must be prepared for pulverization by hand or any other method to avoid damaging the grinder. The process can be quite messy and painstaking, depending on the material an methods used.
Defatting Concerns
Depending on the extraction methods used, foliage will require a defatting phase in order to better facilitate a proper extraction. This generally requires that the material undergo extraction by acidic water prior to defatting. The product will be rendered water soluble and will remain in an aqueous phase as the solution is washed with an NPS to remove fats and oils.
Vendor Considerations
Though none of the plants containing DMT are illegal, the alkaloids contained within generally are. Vendors generally must take it upon themselves not to advertise the illicit contents of their products, otherwise risking putting themselves and their customers in jeopardy. Ordering these botanicals from across borders into countries where their contents are considered illegal is ill-advised but not necessarily risky, especially not if intended for legitimate use. Always be sure to research the reputation of a vendor prior to purchase, as the potentially taboo nature of their products may inspire unethical business practice, such as unreliable delivery or poor product quality; though such cases are not necessarily specifically limited specifically to these sorts of vendors, of course.
Considerations Regarding Cultivation
Again, as these plants are usually not illegal, neither is their cultivation. Often these plants are only native to very specific regions, thus requiring very specific growing conditions, but many can be successfully cultivated indoors or seasonally outside of their native regions. Cultivation for the expressed or deduced purpose of refinery, use or sale of an illicit substance can result in harsh legal consequences, however.
Extraction
Extraction generally refers to the process of isolating a product from a source. The basic idea is to utilize the unique properties of the product—whether reactive, electromagnetic, or otherwise structural—to draw it out of the source and into a target solvent. To accomplish this the product must either be naturally soluble in the solvent or must undergo reaction to increase its solubility. The difference between a high and low yield is firstly determined by how much more soluble the product is in the target solvent, than in its source material or solution, and secondly by how thoroughly the target solvent is mechanically brought into contact with the target solvent; this is generally determined by a combination of the droplet size and dispersion of a solvent and/or the surface area exposure and structural consistency of the source material.
See also:
Straight-To-Base Extraction
Straight-To-Base or STB techniques are generally considered the simplest of extraction techniques and, as such, are the most commonly used. The process involves the use of a strong base reagent in solution to break down source material and convert the contained product from its natural salt form to freebase, which will in turn be more soluble in an NPS than in the basic aqueous solution.
Considerations:
- The use of "Straight To Base" techniques requires little experience or technical know-how for beginners to approach extraction techniques. STB is best-suited for quick, non-labor-intensive, crude bulk extractions. It requires no straining or cooking but requires time for soaking and separation. STB tends to yield a greater array of botanical impurities due to its lack of straining and defatting. These techniques do however enable a more thorough exhaustion of product from the material. This technique is ideal for shredded material that requires little or no defatting.
Overview of Materials and Methods
| Materials Required | |
|---|---|
Source Material:
|
|
Solvents:
|
|
Reagents/Desiccants:
|
|
Equipment:
|
Material Considerations:
- Lye is extremely toxic and hazardous, though most find it easy to handle safely.
- STB methods generally demand a hefty volume of solvents for sufficient performance.
- Naphtha and heptane are generally found to pull a more pure product, and though their poor ability to dissolve DMT demands some amount of heating to pull a considerable amount, it also allows for product to be isolated by freeze-precipitation.
- Toluene and Xylene are generally found to dissolve DMT more easily but also dissolve a wider range of alkaloids and other substances from the source material, and though they do not facilitate freeze-precipitation, they do facilitate the use of FASA.
- DCM is capable of pulling an even fuller range of alkaloids from the source material, as unlike other NPS's, it sinks in water.
- Diethyl ether as a binary solvent in combination with heptane also pulls full-range alkaloids. Using this combination the ether can be distilled off rather rapidly, leaving behind an alkaloid-saturated solution of heptane ready for immediate freeze precipitation. Note! Diethyl ether is extremely flammable. Exercise extreme caution!
- Limonene has become the solvent of choice among many--largely due to being nontoxic--but is considerably more expensive and harder to find than other less savory NPS's, and though it does not facilitate the use of FASA or freeze-precipitation, it can facilitate salting methods like FASW and FASIPA.
- Most commonly used solvents do not facilitate expedient or clean evaporation, due to either impurities from the solvent itself or pulled from the source material, though heptane and naphtha tend to be most effectively used in this way.
Methods:
- STB extraction involves a soak of pulverized source material in a basic solution, followed by the stirring in of an NPS. The NPS is separated from the aqueous solution by siphoning, pipetting, or use of a separatory device.
Material Preparation
For standard STB, the source material must generally be at least shredded, though preferably powdered. It has been found beneficial to pre-treat the pulverized material with an acid soak, with or without heat, prior to immersing in a basic solution.
Extraction Procedure
- Prepare a basic solution between pH 12-14 by adding base to the appropriate amount of water for the amount of source material to be used.
- Immerse the pulverized source material in the basic solution and allot time for material breakdown and freebase conversion.
- Stir or shake in the target solvent enough to ensure adequate dissolution of product.
- Allow the solvent to separate from the aqueous solution in two distinct layers and separate using appropriate separatory methods.
- Collect solvent and proceed with appropriate desired crystallization methods.
Further Elaboration and Technical Support
Acid/Base Extraction
Acid/Base or AB extractions use a weak acid(citric, acetic, and OTHERS have been used with success) to extract the DMT from the plant material into DMT freebase.
Considerations:
- The use of Acid/Base techniques implies the use of "acid-cooking" the source material, straining it, and basifying the resulting strained solution. The use of an initial acid extraction facilitates the implementation of a defatting phase and generally yields a product more devoid of botanical impurities. This technique is ideal for any material that requires defatting, though defatting may not be necessary, depending on the intended method of crystallization.
Overview of Materials and Methods
| Materials Required |
|---|
Methods:
Material Preparation
Either purchase shredded bark or Break 1-Pound of Mimosa Hostilis rootbark into 2" pieces and grind it all up in a glass-topped blender, a little at a time.
Extraction Procedure
(taken from tek) Premix in an empty 1-Gallon plastic jug: 1-Pint White Vinegar & 3-1/2 Quarts Water. Put the ground up Mimosa in a 3-Liter crockpot, then fill it with the water-vinegar solution.
Stir well and turn it on "high". After 2 hours, remove the crockpot ceramic liner, hold the lid on slightly offset, and pour off most of the liquid into a 1-gallon wide-mouthed glass or stainless container.
Add the remaining water-vinegar solution to the crockpot again. Stir well and turn it on "high". After 2 hours, remove the crockpot ceramic liner, hold the lid on slightly offset, and pour off all of the liquid into the same container again. Discard the rootbark fiber and save the two combined extractions in the 1-gallon container.
Allow the vegetable particles in the extraction in the 1-gallon container to settle to the bottom overnight. Then pour off the liquid into an empty 1-Gallon GLASS wine jug, being careful not to pour off any of the vegetable sludge at the bottom. Discard the sludge and keep the contents of the wine jug.
Premix in advance a solution of: 4 Tablespoons (50grams) of Sodium Hydroxide ("Red Devil" lye) with 1-Pint of HOT Water. Stir well. Slowly add this solution to the wine jug, then cap the jug. Gently tilt the wine jug back and forth for 1 full minute to mix the contents.
from here use your solvent of choice
Further Elaboration and Technical Support
Dry Technique Extraction
| Note: | This technique is still experimental and may result in variable success if used with MHRB. |
Dry techniques (drytek) evolved from and are ideally intended for the implementation of the FASA method of crystallization and serve as the only techniques able to implement acetone as an extraction solvent. Acetone is generally favored for its ability to extract a notably broad range of active products.
Considerations:
- The use of dry techniques requires fewer and less toxic materials than the techniques that employ aqueous phases and separatory methods. The materials required are generally of a more household nature. They are most effectively applied to powdered botanical material. Acetone is, however, completely water miscible, so proper drying procedures are of the utmost importance. This technique may or may not require the defatting of botanical materials, depending on the intended method of crystallization. Dry techniques are the youngest of the current extraction techniques though apparently sound in theory and in practice.
Overview of Materials and Methods
| Materials Required | |
|---|---|
Source Material:
|
|
Solvents:
|
|
Reagents/Desiccants:
|
Material Considerations:
- Acetone can be purchased at hardware stores but should be confirmed as pure acetone prior to purchase. Note that almost all acetone can contain up to 5% water contamination, depending on time and shelving conditions.
- In order to be utilized for extraction, sodium bicarbonate must undergo conversion to sodium carbonate.
- Lime is often found difficult to decant acetone off of and also difficult to filter out of acetone, whereas sodium carbonate is generally found more agreeable for both.
- Distilled water is preferable, as tap water almost always contains impurities that can potentially tamper with resulting yields.
- With few exceptions, the source material should be completely pulverized to a powder consistency before use, as this technique's choice of reagents are not quite capable of penetrating cell structure.
Methods:
- Extractions by dry techniques are characterized by the lack of a traditional aqueous phase in the extraction process, and instead, opting for basification within a paste which is followed by chemically drying the paste with desiccant. The process does not make use of separatory methods, and instead is characterized by it's use of dry-washing, decanting and non-intensive filtering methods. Certain materials must be rendered anhydrous prior to use.
Material Preparation
| Rendering Anhydrous Magnesium Sulfate | ||||
|---|---|---|---|---|
|
| Rendering Anhydrous Acetone | |
|---|---|
|
| THP Approach to Rendering Anhydrous Acetone | |
|---|---|
|
| Conversion of Sodium Bicarbonate into Sodium Carbonate | ||||
|---|---|---|---|---|
|
Extraction Procedure
- Mix the intended base with the powdered source material at a ratio between 1:2 and 1:1.
- The product remains in its natural salt form which is generally considered to be quite free from the botanical cell structure in powdered material.
- Add only enough water to thoroughly moisten the mixture to the consistency of a paste while stirring to ensure the consistency of the mixture.
- Although this is not generally considered a traditional aqueous phase in that it is not a solution, it is an aqueous phase in that it is excessively hydrated and sufficiently aqueous to facilitate reaction.
- Allow adequate time to soak in order for reaction to occur.
- The acid component of salt-form product undergoes reaction with the base, effectively neutralizing the acid and freeing the product in its pure alkaloid form, or freebase.
- Stir in anhydrous magnesium sulfate until thoroughly dry.
- The magnesium sulfate acts as a desiccant, and that this is performed in order to prevent water contamination of the acetone.
- Add an excess of anhydrous acetone and stir thoroughly, allotting adequate time and stirring for thorough dissolution of the product into the acetone.
- The more contact allotted between the product and the acetone, the greater the saturation.
- Decant and/or filter acetone and collect, being careful not to allow any particulates into the collection vessel.
- The bases used should not harm the quality of the product, but may interfere with the accuracy of weight.
- Repeat steps 5-6 with fresh acetone until material is exhausted to satisfaction, and proceed to the appropriate desired crystallization method.
- Three washes is generally considered sufficient.
Further Elaboration and Technical Support
Limtek Extraction
Though all of the other extraction techniques may be employed in nontoxic or at least less toxic manners, few are perfectly suited for completely nontoxic implementation with food-grade household chemicals. Limtek extraction is named as such because it employs the use of d-Limonene and pickling lime, and is distinguished by the unique way in which lime must be used for effective results--similarly to drytek--as well as the hygrophobic properties of limonene. Unlike most bases used in extraction, lime has very low solubility in water, and so even though it does qualify as a strong base, it does not behave as such in solution; it must be mixed into a paste with the source material and a minimal amount of water in order to behave as a strong base. One of the drawbacks of drytek extraction is that upon removal of moisture from the mixture of material and reagent, the reaction will essentially terminate, greatly limiting the effectiveness of the extraction. Limonene, however, is an NPS and so is hygrophobic, meaning that the source material can remain moist.
Considerations:
- Limtek extraction is nontoxic and food-grade throughout, using no toxic or otherwise hazardous materials in the process. This technique uses the absolute minimum of material and volume possible for extraction, and due to it's lack of a proper aqueous phase in the extraction process, requires no separatory methods prior to salting and facilitates solid disposal in lieu of dumping large volumes of potentially toxic solution. This process bears the least resemblance to the production of other less savory substances, reducing legal risks to the operator, to include chemical odors.
Overview of Materials and Methods
| Materials Required | |
|---|---|
Source Material:
|
|
Solvents:
|
|
Reagents/Desiccants:
|
Material Considerations:
- In order for the process to remain nontoxic, an aqueous food-grade acid should be used to salt out of the product.
- FASW (Fumaric Acid Saturated Water) can be evaporated to achieve a crystalline DMT fumarate.
- vinegar can be used to achieve DMT acetate but will not crystallize.
- Limonene can be replaced with other NPS's, but the process will cease to be nontoxic.
Methods:
- Extraction by limtek is characterized by the lack of a proper aqueous phase but also the lack of necessity for drying procedures, and essentially involves a pasty or doughy material being washed in the target solvent to retrieve product.
Extraction Procedure
- Mix the powdered material with lime between 1:2 and 1:1 and mix thoroughly.
- no reaction occurs at this point but thorough mixing will help to ensure an even reaction for the following steps.
- Thoroughly moisten the mixture while stirring, ensuring that no dry spots remain and the mixture exhibits a doughy or pasty consistency.
- the added water facilitates reaction and a basic environment which may aid in breaking down the plant material.
- the acid component of the natural salt-form of the product within the plant material will be neutralized, yielding a freebase product.
- Ensure a thoroughly homogeneous mixture and allot enough time and stirring for a complete reaction.
- this is the most important part for a successful yield, and due to the lack of a proper aqueous phase, the reaction requires a stronger degree of manual facilitation.
- Wash the mixture with NPS by stirring and decanting to collect the NPS, and proceed to the appropriate desired crystallization method.
- the more contact brought between the material and the target solvent, the more successful the extraction will be.
Further Elaboration and Technical Support
- Directory of Current Limteks
- A Poll to Gauge Members' Success w/ Lime
- Pickling lime instead of lye?
- Calcium Hydroxide instead of lye?
Crystallization
Crystallization is the process by which a product is isolated from a solvent. This is accomplished by either allowing the solvent to completely evaporate or by causing a precipitation to occur within the solvent, which can then be isolated from the solvent by several methods and then dried of any residual solvent.
Evaporation and Slow Precipitation
In extraction, evaporation is the process by which a solvent disperses from its liquid form into the air as a vapor and a gas. When this occurs, the less volatile constituents of the solvent solution are left behind, and as such, it is a common method of isolating solutes from solvent. Typically, a solvent is evaporated in a shallow dish in order to maximize surface area exposure, often with mild heat and airflow applied to hasten the process; however, minimizing surface area and airflow and eliminating heat are often found to improve the quality of precipitate yielded by process of slow precipitation. Many solvents are found to be limited in their ability to crystallize a product by evaporation, yielding a product ranging from oils and "goo," to waxy crystals, to fluffy crystalline powder, to hard crystal shards. These discrepancies in quality of yield are determined by the solvents speed of evaporation, solubility of product in the solvent, or impurities present in the solvent.
Considerations:
- Many common solvents contain impurities which may not be quite as volatile as the pure solvent and may leave these impurities behind as a residue.
- Solvents often emit fumes and odors which may be hazardous to health, flammable, or may alarm those within proximity of the odor.
- Some solvents may require an excessive length of time to evaporate.
- Some solvents may absorb ambient moisture, resulting in a less expedient evaporation.
- Excessive air flow may cause the oxidization of the product.
- Solvent may become Trapped within the crystal structure of the product, resulting in a less solid and less pure product.
Full-Range Extracting Solvents
Low-Solubility Solvents
Theses solvents dissolve a much more narrow range of product and are used to isolate DMT from active and inactive impurities, alike. However, when used for extraction, they are often heated and dissolve a bit more variance in alkaloids, and they may require further purification.
- Heptane requires much heating to dissolve any significant concentration of product but precipitates product very easily at room temperature or cooler. It is often found in quite pure form, reliably free of additives, and is a choice solvent for recrystallization and slow evaporation and precipitation.
- Naphtha can require a bit of heating to dissolve a significant concentration of product but precipitates product relatively easily at room temperature or cooler. While never without a wide variety of additives, some brands will evaporate cleanly without leaving a residue. This is a common solvent used for both extraction and evaporation but is often considered most preferable for freeze precipitation.
Polar Solvents
Freeze Precipitation
Freeze precipitation is the process by which product is isolated from a solvent through a decrease in solubility achieved by lowering the temperature of the solvent. This process generally relies on the solvent being completely saturated or super-saturated with product. Freeze precipitation is generally the fastest method by which product can be isolated immediately following extraction, but it relies on the use of only very specific solvents. This method is preferably used in conjunction with A/B and STB techniques though can be used alongside variations of drier techniques.
Manual Crystallization
| Manual Crystallization[1] | ||||
|---|---|---|---|---|
|
Salting
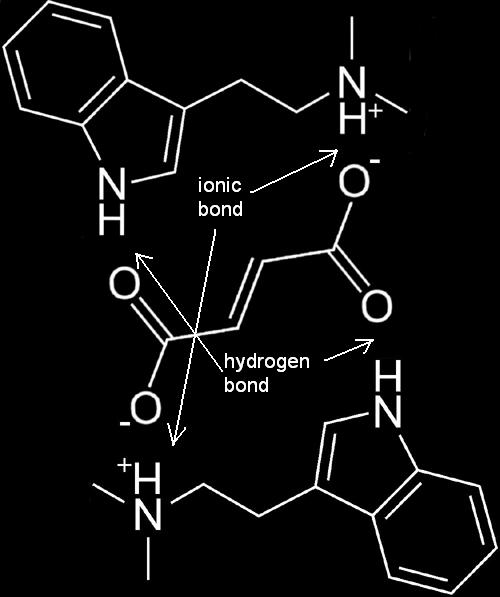
Salting is the process by which freebase DMT is reacted with an acid to create a salt form which is generally water-soluble. The natural form of DMT in botanical sources tends to be a salt-form, thus facilitating the simple aqueous extraction used to prepare DMT-containing brews. It is quite common to perform aqueous acid extractions from the material, however—whether for the purposes of a brew or for A/B extraction. The salt-form itself rarely lends itself to proper crystallization and usually can only be isolated as an oil unless very specific methods and materials are employed.
The Use of Fumaric Acid
Few salt-forms of DMT facilitate crystallization, but DMT fumarate currently stands alone as a solid crystalline salt-form of DMT.
The FASA Method
| Materials Required | |
|---|---|
Source Material:
|
|
Solvents:
|
|
Reagents/Desiccants:
|
The FASA, or fumaric acid saturated acetone, method is a method employed to render DMT Fumarate.
Considerations:
- DMT Fumarate is reportedly quite stable and resistant to oxidization or other forms of degradation. It is notably resistant to heat, and as such is able to withstand low-temperature oven-drying. Certain other related compounds, such as jungle-spice and bufotenine are also able to crystallize as a fumarate. Defatting is not required prior to employing FASA methods, as oils and most other impurities should not interfere with this method's procedure or the yield
- Because DMT Fumarate is water-soluble, it is also well-suited for oral administration in conjunction with harmaloids, either mixed into a beverage or encapsulated.
Methods:
- The FASA method employs the firstly, the solubility of fumaric acid in acetone, and secondly, solubility of freebase DMT in acetone, and thirdly, the insolubility of DMT Fumarate in acetone or the non-polar solvents commonly utilized for extraction. The solubility of both DMT and fumaric acid in acetone facilitates their reaction to produce a crystalline DMT salt which is completely insoluble in acetone or non-polar solvents.
FASA Molecule
One fumaric acid molecule attaches to two DMT freebase molecules.
Procedure
| Rendering Crystalline DMT Fumarate | |
|---|---|
|
Links
- The FASA Method: A Summary - DMT Fumarate and Beyond
- DMT Fumarate to DMT Freebase
- FASA Alteration of Final Purification
- Fumarates to Freebase Conversion TEK
- Spice Extraction-The FASA Approach
The FASW Method
this is a procedure to salt a 100-300ml pull of limonene the measurments are more of a rough guideline it doesnt have to be exact play around with what works best for you... Get a small jar/beaker/cylindrical container and put around 300ml of WARM water in it. Add ~2g of fumaric acid to it. Stir this like crazy. There may be a few fumaric acid chunks in there but can leave them and fill with more warm water for later pulls.
Stir 50ml FASW into the d-limonene for at least 10 minutes, HARD. Then suck out/drain off the dmt fumarate saturated water, which will be the bottom liquid layer. SWIM usually cleans out the third small jar and puts this saturated water in it before putting the water on the evaporating plate. This is so that he can filter the water before it hits the evaporating plate. This keeps any traces of d-limonene out of the soon to be evaporated dmt water. Repeat this step two or three more times with 50ml fresh FASW each time per pull of limo
Evaporate the saturated dmt water in the dehydrator or on a very very low oven setting, no more than 175F. This will result in the solid DMT fumarate. This stuff is HARD. It may be difficult to scrape up, but just use some muscle and it will come up. from here you can freebase or just eat the fumarate like i do ;]
Note: the solubility of fumaric acid in 100ml water is 0.63 g @ 25°C, 1.07 g @ 40°C, 2.4 g @ 60°C, 9.8 g @ 100°C. (The Merth Index, 8th ed.)
The FASIPA Method
Take your small jar and put 5g fumaric acid in it and then fill the jar with your 99% IPA. Then mix this together until the IPA is completely saturated. This creates Fumaric Acid Saturated IPA(FASI). Most likely you will have excess fumaric acid on the bottom but that is fine. Now SLOWLY pour your FASI into your jar of dmt saturated d-limonene. The slower the better. For best results add 10ml FASI every 5 minutes until you dont see any more cloud forming in the d-limonene. This will crash out your dmt from the d-limonene. Allow this to sit for at least 24 hours after you stop putting in FASI so that the DMT Fumarate can fully crash out. After 24 hours add another 1ml of FASI to make sure all dmt has precipitated out. After you collect the DMT fumarate from the jar, put the d-limonene back into the jar and let it sit for a few days. More DMT fumarate will precipitate over time. Now pour off the d-limonene from your now beautiful crystals. Turn the jar upside down and put it on a paper towel to absorb any extra D-Limonene that drips out. Allow this to sit out until it dries a bit more. You can use a bit of heat and a fan to speed this process up. Now scrape up your JIMJAM fumarate. It will smell mildly of oranges. DONE
Discussion of Other Salt-Forms
Further Elaboration and Technical Support
Purification
The purification of DMT product has several purposes and is accomplished by several different methods, but all of them essentially involve the washing of product in some way or another. Purification either involves the isolation of product from unwanted impurities from the plant source or from the process of extraction, or it involves the isolation of product from active impurities which may or may not be collected after isolation.
Recrystallization
The general purpose of recrystallization is to crystallize the product in a fresh solvent after it has already been isolated from the extraction solvent, likely containing a considerable amount of impurities. In this technique, an impure solid compound is dissolved in a solvent and then allowed to slowly crystallize out as the solution cools. Often this process results in more well-formed crystals with less discoloration. The advantage of this method of purification is that the solvent choice for recrystallization may be different and more suitable than that chosen for extraction. Crystallization of a solid relies on slow, selective formation of the crystal lattice and is quite different from precipitation. In freeze precipitation, there is a rapid formation of a solid from a solution that causes impurities to be trapped within the solid's crystal framework. For this reason, extractions that rely on precipitation or evaporation to produce a solid product always include a final recrystallization step to give the pure compound.
The process of recrystallization relies on the property that for most compounds, as the temperature of a solvent increases, the solubility of the compound in that solvent also increases. For example, much more sugar can be dissolved in very hot water (just below boiling) than in water at room temperature. Inversely, if a hot saturated solution of sugar and water is allowed to cool, sugar will begin to crystallize out of solution as solubility decreases. Recrystallization will give your product a more sharply defined, uniform melting point and in the case of DMT allow for hard non-waxy crystals.
| Removing Inactive Impurities via Recrystallization | ||||||
|---|---|---|---|---|---|---|
|
Finding a suitable solvent
The first consideration in purifying a solid by recrystallization is to find a suitable solvent. A good recrystallization solvent should fit the following criteria:
1. The compound should be very soluble at the boiling point of the solvent and only sparingly soluble in the solvent at room temperature.
2. The unwanted impurities should be insoluble in the hot solvent.
A good recrystallization solvent for DMT is heptane. DMT is not very soluble in it at room temperature but quite soluble as we add heat. Most common spice impurities, however, are not very soluble in it at all and can thus be separated via simple decanting.
Dissolve solid into hot solvent
Prepare a waterbath and heat the DMT and the heptane in their own beakers until the DMT begins to melt. Add heated solvent dropwise into the beaker containing the extract. The heptane will go cloudy almost immediately and take on a yellow color as the DMT goes into solution. Keep adding heated solvent until further addition or agitation causes no more DMT to dissolve.
Your beaker should now contain yellowish-tinged heptane with an orange-brown blob of oil and undissolved solids at the bottom of the vessel. Carefully decant the solution into another beaker, careful to leave the impurities behind. Repeat dropwise addition of heated solvent and decantation to ensure no DMT is left behind.
Decolorize with carbon
Now that we have dealt with insoluble impurities, our solution is see-through but tinged yellow. This discoloration is due to the presence of high-molecular-weight reaction by-products which may have been formed during the extraction process. A simple wash with activated carbon will get rid of decolorizing compounds. (Activated carbon is extremely efficient at absorbing impurities due to its large surface area.)
1. Add excess solvent and activated carbon, and boil the solution for a few minutes. The colored impurities will adsorb onto the surface of activated charcoal.
2. Remove the charcoal with absorbed impurities by filtration. Your solvent should now be almost clear. If the yellow color persists, repeat the charcoal wash carefully.
Note: Very little activated carbon is needed to remove the colored impurities from a solution. You must be careful in your use of decolorizing carbon: if too much is used, it can adsorb the desired compound from the solution as well as the colored impurities.
Crystallization
After the solution has been filtered cover the flask containing the hot filtrate and set it aside undisturbed to cool slowly to room temperature. As the solution cools, the solubility of the dissolved compound will decrease and the solid will begin to crystallize from the solution. Once the solution reaches room temperature, move it into the refridgerator, and finally into the freezer to freeze precipitate most of the DMT.
The slower your solution cools, the cleaner and larger your crystals will be.
Further Reading
How to Crystallize Organic Compounds - WikiHow
Recrystallization Technique - Rhodium
Washing Spice
The purpose of washing is to disperse impurities off of the product or out of a solution containing the product and into an intermediate solvent.
Alkaline Solution Washing of Impurities
Most of the impurities that plague yields tend to be quite soluble in both alkaline aqueous solutions and non-polar solvents. To remove these impurities, an imbalance in equilibrium must be created between these two types of solutions, causing the impurities to disperse into a disposable solution from the solution containing the product. This procedure is commonly used for purifying product from extractions that utilize naphtha or heptane to obtain a whiter product.
| Washing Spice of Oxidization and Inactive Impurities Using Alkaline Solution[2] | |
|---|---|
|
Solvent Washing and Isolation of Impurities
Some product may contain undesirable active or inactive impurities with solubility properties differing greatly from the more desirable components.
| Purification of Spice Fumarate Product[3] | ||||
|---|---|---|---|---|
|
Freebase Conversion from Salt
The methods used for converting crystalline salt-form DMT into freebase are not dissimilar from those used in extraction. The only significant difference between the processes is that the conversion involves far fewer impurities and less material than the extraction. Because of of this and the fact that it involves the isolation of the product from an acid, the conversion acts as a sort of purification method.
Drytek Conversion
This conversion is preferred for it's lack of need for separatory methods and for it's notably dry quality which facilitates the use of acetone. This method of conversion evolved out of the FASA method and is characteristically identical to Drytek extraction.
| Drytek Freebase Conversion of DMT | |||||
|---|---|---|---|---|---|
|
This method is the most common way to achieve a usable freebase product. Though it is advisable to keep everything completely free of moisture in this process, impurities carried through by moisture are not dangerous and merely effect weight.
|
Crystalline Conversion
| Crystalline Freebase Conversion of DMT | |
|---|---|
|
Though heptane is a bit more toxic and more difficult to evaporate than acetone, it is able to achieve a more pure, hard, crystalline product. The only likely potential impurity--assuming proper decanting--is residual heptane.
|
Nontoxic Freebase Spice Conversion
Nontoxic Hasty Freebase Spice Conversion
| Nontoxic Hasty Freebase Spice Conversion[4] | ||||||
|---|---|---|---|---|---|---|
|
This method is the most expedient and simplest method of freebase conversion.
|
Nontoxic STB Freebase Conversion of DMT
| Nontoxic Freebase Conversion of DMT[5] | |
|---|---|
|
Though completely nontoxic, this method is reportedly difficult for achieving a manageable product.
|
STB Conversion
This conversion is simplistic in that it almost exactly resembles the methods used in STB Techniques, though it is significantly simpler in that it involves less material, fewer impurities, and does not require a strong base.
| Materials Required | |
|---|---|
| Source Material: |
|
Solvents:
|
|
Reagents/Desiccants:
|
Procedure for STB Freebase Conversion of DMT:
- Dissolve DMT Fumarate in an adequate amount of water.
- No reaction occurs at this point.
- Add a concentrated solution of weak base until total precipitation is observed by the cloudiness of the solution.
- The fumaric acid undergoes reaction with the base, effectively neutralizing the acid and freeing the product in its pure alkaloid form, or freebase.
- Stir in NPS thoroughly and allow to separate from the aqueous solution.
- The more contact allotted between the product and the NPS, the greater the saturation.
- Collect the top layer of NPS, being careful not to allow any aqueous contamination.
- Aqueous contamination may result in an impure product or may disrupt subsequent crystallization.
- Repeat steps 3-4 with fresh or unsaturated NPS until the aqueous solution is exhausted to satisfaction.
- Three washes is generally considered sufficient.
- See Crystallization in order to render crystalline freebase product.
Further Elaboration and Technical Support
Administration
Leaf Enhancement
Enhanced Leaf is a method of administration whereby one dissolves their DMT freebase in either Ethyl Alcohol, Acetone or Isopropyl Alcohol with any smokable herb and allowing this to evaporate. This allows an easier administration than freebase DMT, a cone is loaded and pulled very slowly, being careful not to pull it too fast, as this will not vapourise the DMT and alot of the DMT will be wasted.
Procedure
Thoroughly dissolve the freebase DMT (use about 40ml of solvent per gram of DMT)in the warm solvent of choice. You want to use a dish with a width that will allow your leaf to sit between 5-10mm high. Add the leaf to the DMT saturated solvent and swirl it around so all the leaf is spread evenly on the bottom of your dish. If needed, a little more solvent can be added so that the leaf is well covered. Place the dish somewhere where it will stay undisturbed for several days. Once completely dry, the leaf can be weighed. It should be heavier by the amount of freebase DMT that it is enhanced with.
Changa
"Changa is a smoking mixture containing similar ingredients to the Shaman's medicinal brew Ayahuasca.
Although there are many varieties of Changa, like Ayahuasca the key active ingredients are consistently DMT and an MAOI.
The effects of Changa are considered by many to be more grounded than just DMT freebase smoked on its own. The effects have been reported as being similar to a short Ayahuasca trip. The duration of effects is slightly longer than that of freebase DMT, with reports of trips lasting up to 12 minutes. There have also been reports of Changa being used in conjunction with Ayahuasca to intensify and then later revisit the experience.
Changa is a lot easier to use than freebase DMT, and can be smoked through an ordinary pipe or water pipe. This is especially important, as DMT freebase can be typically difficult to vaporise on its own.
The percentages of DMT and MAOI concentration in the mixture are variable. A typical mixture would be characterised by breakthrough experiences at a dosage of approximately one pipe. There have also been reports of breakthroughs occurring with Changa that has been rolled into joints."[6]
Herbs
- Pau D'Arco: Rich and exuberant meadows of pleasure
- Blue/White/Pink Lotus: A distinct pleasurable and bright character
- Calea zacatechichi: The dream herb allows for a perfect synergy
- Passionflower: MAOI which potentiates and lengthens the experience
- Banisteriopsis caapi: Adds a spirit quality and potentiates and lengthens the experience
- Damiana: Unique vibrance and taste
- Brugmansia Flowers: Distinct other wordly character
- Silene capensis: African dream herb
- Peppermint: Adds fresh soothing qualities
- Mullein: Respiratory healing and soothing qualities
- Heimia salicifolia: Increases auditory hallucinations
- Justicia pectoralis: Smoother taste and a nice smell
- Salvia divinorum: Acts as an opener and enabler
- Chaliponga: Adds a spirit quality and more magic
- Mugwort: Dream synergy and mystical powers
- Pedicularis densiflora: Psychoactive sedating effects
- Sculcap: Strengthens, calms and sedates nervous system
- Wild lettuce: Strengthens, calms and sedates nervous system
- Lions tail: Euphoric smoking herb
- Synaptolepis Kirkii: Adds a clear minded, visionary quality
Miscellaneous
Additives
- Harmala Extract
- Bufotenine
- 5-MeO-DMT
Blends
- Ayahuasca Android: 70% Caapi - 30% Chaliponga
- Dream Scape: 60% Calea - 40% Brugmansia
- Electric Sheep: 50% Blue Lotus - 50% Calea
- Minty Blast: 40% Capi - 35% Peppermint - 25% Mullein
- The Mekong: 50% Passionflower - 50% Blue lotus
- Twisted Fruit: 50% Caapi - 20% Salvia - 30% Mugwort + Bufotenine
- Xnga blend
450mg peppermint 450mg Caapi leaf 800mg Washed, recrystallized Freebase DMT 300mg Freebase Harmala (extracted from Caapi vine or Syrian Rue) Spiritualitymg Anhydrous Fairydust.
- Witch Drum:
1000mg herb blend consisting of 40% Blue Lotus 40% Calea Zacatechichi 20% Passionflower 300mg Freebase Harmala Alkaloids + 1000mg Freebase DMT
Vaporization
The vaporization method of administration pertains to the use of pure freebase product with no additional material. This method generally makes use of heating an apparatus that is intended to distribute the heat to the product until it reaches the point of vaporization and can be inhaled.
Glass-Pipe Vaporizers
Glass-pipe vaporizers are glass pieces that are meant to be heated directly in order to indirectly disperse that heat into the product. The glass used must be thin enough for the heat to pass through its structure and potentially distribute the heat evenly. The piece must have a chamber within which the product will undergo vaporization. It must be assembled in a manner that will allow for air intake and output: The output being the inhalation nozzle, and the input being a sort of carb.
| the Improvisation of a Glass Pipe Vaporizer | |
|---|---|
The Machine
"The Machine" is essentially a glass vaporizer in which heat is meant to distribute through the pipe rather than across the glass. It utilizes a metal mesh plug inside of the pipe, on which the product is to be placed, melted and vaporized. The mesh acts both as a screen and a heat-sink, simultaneously allowing for the even heating of the product, protection from the naked flame, and prevention from inhaling unvaporized particulates. The manner of heating is likely to be conduction, as the product is known to run from the heat across the mesh until completely vaporized; however, most designs cause the product to simply run into more heat, resulting in continuous vaporization.
This method of vaporization includes the standard variations—which are essentially the simple combination of a vaporizing bowl and a vapor chamber with an inhalation nozzle—and the bubbler variations in which the vapor passes through a water heat-sink before reaching the vapor chamber and inhalation nozzle.
Material Considerations:
- Copper may gradually turn black due to the formation of Copper(II) oxide (CuO), an inert solid. This reaction and its products are not detrimental to the process of administration.
| the Improvisation of the Standard Variation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Machine-Style Bubbler Piece
| NOTE | |
|---|---|
| Theses variations can apply to the use of a small bubbler pipes, bong-style bubblers, or improvised bubblers, though smaller bubblers are often found most preferable. The water in the bubbler acts only as a heat-sink and will not absorb a significant, if any, amount of product, as the freebase product is not soluble in water; however, the use of ice in cooling should be forgone, as it may cause the premature precipitation of product within the chamber. |
| the Improvisation of the Bubbler Bowl Variation of "The Machine"[12] | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| the Improvisation of the Machine-Style Bubbler Stem[13] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Further Elaboration and Technical Support
Effective Use and Maintenance
As the term indicates, vaporizers are intended to vaporize, not to burn product, as such, the product should never come into contact with the flame or be overly heated to the point of burning. Generally, a vacuum must be generated in order to direct the heat through the product and to direct the vapor into the chamber. Which should not be allowed to sit for too long, as it may begin to precipitate within the chamber. Every toke should be held in the lungs as long as necessary for the vapor to be completely absorbed, as no vapor should be exhaled.
Heat-Source:
- Though many prefer a butane torch for expedient heating, and though many manners of vaporizer demand its use, a standard lighter will produce adequate heat for vaporization. A standard lighter is generally ideal for vaporizers that generate a strong enough vacuum to pull the flame toward the heat sink.
Common Methods of Inhalation:
- Some prefer to vaporize their entire dose before inhaling so as to administer one strong toke. This method can be quite harsh and difficult on the lungs and throat and may induce coughing. However, this is reportedly the most intense method of administration, almost always inducing a "breakthrough" if held in the lungs for adequate absorption.
- Others prefer to administer one dose within multiple tokes, which is reportedly slightly longer-lasting and much easier on the lungs and throat though possibly less intense. Usually the amount of tokes taken depends on the intended depth of the experience. In many cases, three is found to be more than adequate and four is said to be a "breakthrough" dose. Any subsequent tokes to the first toke must occur within the first minute, due to both tolerance and lack of motor skills building up rapidly
Cleaning the Apparatus:
- The apparatus used for vaporization can be cleaned using the same solvents used for extraction. However, volatility and toxicity concerns should be a strong consideration in choosing the proper solvent.
- It is preferable to choose a solvent that evaporates quickly and cleanly and able to dissolve a broad range of products, as oxidization is likely to have occurred.
- Residual solvent residue may be hazardous, as the user may inhale harmful fumes or potentially ignite the solvent.
- The residual product dissolved by the cleaning solvent may be salvaged by appropriate methods of crystallization. However, it is likely to contain a variety of inactive constituents, so proper purification measures would be advisable.
Concerns Regarding the Experience
- The primary effects of smoked DMT, induced within minutes of administration, last for about 5-10 minutes and are characterized by strong visual hallucination, a strong psychedelic quality, and minor auditory hallucination, while the secondary effects of a much more mild character may last for up to an hour.
- Tolerance is reportedly induced rapidly and often dissipates rapidly, though repeated or high doses may induce a slightly longer term tolerance. Some believe it is best to wait at least one hour between doses.
- The experience of smoking DMT is said to be quite overwhelming. It is often necessary for the user to have a "sitter" nearby to tend to the smoking apparatus so as to not risk the possibility of the user damaging it or accidentally inflicting burns. It can also be temporarily debilitating, so the user may be advised to refrain from any physical activity.
- Set and setting are of the utmost importance, as the user is often rendered temporarily vulnerable and emotionally fragile. The user must determine the appropriate conditions in which administration is to take place but is advised to initially seek out a setting containing minimal stimuli.
- It may also behoove the user to seek out a more experienced sitter for guidance and support. Often this is unavailable, and as such, it would be more advisable for the user to perform the rite in solitude. An inexperienced sitter may inadvertently induce anxiety in the user out of negligence or unnecessary concern.
Further Elaboration and Technical Support
Potentiation
- The effects of DMT, as with most psychedelic substances, can be lengthened, strengthened, altered, or otherwise potentiated through the use of various psychoactive or bioactive compounds. The potentiation of DMT is actually the oldest method of administration, as its necessarily potentiated oral administration serves as the compound's longest known history of use. DMT is generally considered to be orally inactive without some form of potentiation, such as a harmaloid preparation. DMT's potentiation is not limited to oral use, however, as many of the same potentiating agents may be used in conjunction with vaporized or otherwise administered DMT.
Harmaloids
Oral Administration of DMT
Pharmahuasca
Pharmahuasca is a pharmaceutical version of the entheogenic brew ayahuasca. Traditional ayahuasca is made by brewing the MAOI-containing Banisteriopsis caapi vine with a DMT containing plant, such as Psychotria viridis. Pharmahuasca refers to a similar combination that uses a pharmaceutical MAOI instead of a plant.
- A general rule for the dose taken of DMT in a capsule is NO MORE THAN 1mg per pound of body weight.
- This is assuming that approx 100mg MAOI is taken first.
- Take one MAOI capsule (100mg) wait ten-twenty minutes
- Take one DMT capsule containg half of the dose (for 150lbs. bodyweight, no more than 75mg DMT)
- Wait ten minutes, this will reduce the likelihood of vomiting.
- Take one DMT capsule containing the second half of the dose.
- Experiment with how much spice SWIM likes to use and find what's right for SWIM.
WARNING
- This method of administration can easily get out of hand by believing a small change in the weight of the dose will have little effect, the above dosage is considered to be a high one. Do not attempt to operate machinery during the trip. Do none of these things, after all SWIM will probably be lying on their back for a couple hours.
Jungle Spice
Jungle spice is one of many names that have been applied to an intriguing non-DMT alkaloid fraction that can be isolated from much of the commercially available Mimosa root bark (See V. Botanical Confustication).[14],[15],[16] In the most general terms, it is the alkaloid fraction obtained from the aqueous basic phase of an extraction by pulling with xylene or toluene after DMT ceases to be pulled by an aliphatic hydrocarbon solvent (naphtha, heptane, etc.). This product will almost always contain some N,N-DMT in addition to the more mysterious alkaloids; some extractors choose to remove the DMT in a hot naphtha wash to obtain a pure "jungle" experience, while others use the jungle/DMT mixture as it is.
There is a great deal of ambiguity surrounding jungle spice, owing to a wide array of factors. First and foremost, there appears to be a great diversity of compounds which can be isolated by extracting the aqueous basic phase with xylene or toluene.[17],[18] Which compounds are actually isolated depends on some several of the following factors: the source and botanical identity of the root bark, the conditions of cultivation/harvest, and various pH, temperature, and airflow considerations throughout the extraction process.[19],[20],[21],[22] About all that is certain about it at this point is that it contains some psychoactive material that isn't N,N-DMT.
There has been a lot of speculation going around that this compound may be yuremamine, the novel phytoindole isolated from Mimosa Tenuiflora and characterized in 2005.[23],[24],[25] Looking at the evidence, this scenario appears exceedingly unlikely based on yuremamine's known instability to base and speculated instability to heat.[26],[27] It can't yet be ruled out completely, but there remains a substantial body of evidence against this identification. Until an LC-MS of jungle spice emerges with a molecular ion at 477.1 m/z, I think it's safe to assume that yuremamine is not the red alkaloid that has been isolated by home extractors.
Links
- Summary of jungle spice analytical work
- "Jungle Spice" - Mystery Alkaloids of Mimosa Root Bark at the DMT-Nexus
- Entropymancers "Jungle Spice" - Mystery Alkaloids of Mimosa Root Bark.pdf
Further Potentiation
Appendices
Storage Concerns
- DMT should be stored in a cool and dry place, preferably in a sealed container.
Brief overview - What is DMT N-Oxide?
DMT N-Oxide is the oxidation compound of DMT. DMT N-Oxide is also very likely psychoactive, as Shulgin hypothesized..
Oxidation can occur naturally (extended exposure to air) or artificially (by using Hydrogen Peroxide). The rate at which oxidation naturally occurs is unknown, but its suspected oxidation to crystals is often superficial, since long term stored DMT remains solid, though sometimes bit darker and more waxy (and N-Oxide is more often described as an oil).
Chemical and physical properties
For solubility, reconversion back to DMT, etc, check the N-Oxide Chemical and Physical WIKI
Effects
Effects should be similar to DMT when smoked.
Pharmacology, toxicity and general safety
Plants containing DMT N-Oxide
Arundo spp.
Arundo donax Culm and flowers. Ghosal 1972a ref Trout's Notes
Acacia spp.
Acacia caesia In bark. Further details not given. Ghosal et al 1970b Trout's Notes
Anadenanthera spp.
- var. cebil [as Piptadenia macrocarpa] - DMT N-oxide In seeds but not in pods. Amount of DMT-N-oxide not given. Total alkaloid content of 1.5-2.0% determined in some samples: Fish et al 1955 ref Trout's Notes. Material from both Florida and Brazil were used.
- (Haiti) (DMT N-Oxide traces in seeds) Paris et al. 1967
- DMT N-Oxide In seeds but not in pods. Amount of DMT-N-oxide not given. Total alkaloid content of 1.6% determined in one sample: Fish et al 1955 ref Trout's Notes. Used material rrom both Puerto Rico and Brazil.
- Epena - DMT N-Oxide present in Yanoama snuff prepared from Piptadenia peregrina. (Marini-Bettolo et al. 1964 ref Trout's Notes)
- Epena - Snuff prepared, by the Ma-hekodo-teri of the Rio Mavaca from seeds of an Anadenamhera specie.s.
DMT-N-oxide [with Bufotenine, Bufotenine-N-oxide, and DMT]. Marini-Bettolo et al. 1964 ref Trout's Notes
Banisteriopsis spp.
- DMT-N-oxide In stem and leaf: Ghosal 1972a ref Trout's Notes
- DMT-N-oxide in leaf. [26 mg from 1.8 kg] Ghosal & Mazumder 1971 and Ghosal et al. 1971
Desmodium spp.
- Aerial parts (0.033%; 0.21 gm. + 0.12 gm. (latter as chloroform soluble acetate) from 1 kg. of fresh wet material. Banerjee & Ghosal 1969 ref Trout's Notes
- Green Plant (Stem and Leaf) Ghosal 1972a and Ghosal & Bhattacharya 1972 and Ghosal et al. 1971
- Ghosal et al. 1972e (0.12 gm. + 0.02 gm. from 1.6 kg. of dried roots.) Ghosal & Banerjee 1969. Roots (Amount not given.) Ghosal & Bhattacharya 1972 ref Trout's Notes
- Fruit Ghosal 1972a ref Trout's Notes
- Seeds Ghosal & Bhattacharya 1972 & Ghosal et al. 1970b ref Trout's Notes
- leaves (0.18 gm DMT-N-oxide. from 2 kg) Ghosal et al. 1972a ref Trout's Notes
- Stem / leaf DMT-N-oxideGhosal et al. 1970b ref Trout's Notes
- Roots. DMT-N-oxide Minor alkaloid. Amount not given. Ghosal et al. 1972a ref Trout's Notes
- Whole plant (DMT-N-oxide Minor alkaloid) Ghosal & Mukherjee 1964 ref Trout's Notes
- (Mention. Ghosal & Mukherjee 1965) (DMT-N-oxide Amount not given) Ghosal & Mukherjee 1966 ref Trout's Notes
- Stem and leaf of young seedling (0.023% DMT-N-oxide by dry weight; 19% of 0.12% Total alkaloid) Ghosal et al 1972c ref Trout's Notes
- Stem and leaf of mature plant (0.070% DMT-N-oxide by dry weight; 5% of 1.4% Total alkaloid] Ghosal e1 al. 1972c ref Trout's Notes
- Root of young seedling (0.011% DMT-N-oxide by dry weight; 3% of 0.37% Total alkaloid] Ghosal et al 1972c ref Trout's Notes
- Root of mature plant [0.121% DMT-N-oxide by dry weight; 11 % of 1.1% Total alkaloid] [Also, in same paper: 1.8 kg of dried roots yielded 0.18 gm + 0.042gm; i.e. 0.012%.] Ghosal et al. 1972c ref Trout's Notes
- Fruit (green) of mature plant [0.007% by dry weight; 72% of 0.01% Total alkaloid] Ghosal et al. 1972c ref Trout's Notes
- Seeds (ripe) mature plant (DMT-N-oxide trace) Ghosal et al 1972c ref Trout's Notes
- Root, Stem-leaf and Seeds - DMT-N-oxide Ghosal et al 1970b ref Trout's Notes
- Root, stem-leaf and fruit - DMT-N-oxide (Amounts not given) Ghosal 1972a ref Trout's Notes
- Leaf [trace DMT-N-oxide] Ghosal et al. 1972d ref Trout's Notes
- Stems - DMT-N-oxide [3% of 0.008% total alkaloids] (dry weight) Ghosal et al !972d
- Roots - DMT-N-oxide (4% of 0.01% Total alkaloids] {41 mg. from 8.3 kg} (dry weight) Ghosal et al. 1972d ref Trout's Notes
- DMT-N-oxide Minor in roots. (amount not given) Ghosal et al. 1971 ref Trout's Notes
Lespedeza spp.
- var. japonica - DMT-N-oxide in rootbark but not leaves. Minor component. Amount not clearly indicated. Morimoto & Matsumoto 1966 ref Trout's Notes
Mucuna spp.
- DMT-N-oxide In root, stem-leaf and pod. Ghosal 1972a ref Trout's Notes
- DMT-N-oxide In leaf/stem/seed. Ghosal et al. 1970b ref Trout's Notes
- 0.003% DMT-N-oxide in fresh leaves Ghosal et al. 1971 ref Trout's Notes
Extraction teks
Dosages and consumption methods
History of usage
Analysis of DMT N-Oxide
Scientific publications
Other links of interest
- ↑ Amor fati's Approach to Freebase Conversion of DMT
- ↑ Acolon 5's Spice Washing Tek
- ↑ FASA Alteration of Final Purification
- ↑ Amor_fati's_Approach_to_Freebase_Conversion_of_DMT#Nontoxic_Conversion
- ↑ DMT Fumarate to DMT Freebase
- ↑ Reoxy: Changa[1]
- ↑ "The_(mini)_Machine"_Step_by_Step_Guide
- ↑ q21q21's Test-Tube Machine
- ↑ Reintroducing My Alternative to "The Machine" by amor_fati
- ↑ New stealth bargan vapour pipe by deweeb
- ↑ My Machine by Aoutiv
- ↑ Posts Regarding Construction and Operation of a Machine-Style Bubbler Bowl by WSaged[2][3][4][5]
- ↑ The Mini-Machine Bubbler Stem
- ↑ Doctorcito (@ Ayahuasca Forums). 2006. "Yuremamine: Solving the Mystery of Jurema Preta?" http://forums.ayahuasca.com/phpbb/viewtopic.php?t=9912
- ↑ DonPeyote (@ Drugs-Forum). 2006. "Jungle DMT?" http://www.drugs-forum.co.uk/forum/showthread.php?t=26468
- ↑ Lemmiwinks (@ Entheogen.com Forums). 2007. "Red Spice?" http://www.entheogen.com/forum/showthread.php?t=11191
- ↑ Fuego (@ DMT-Nexus Forums). 2007. "Doing xylol extraction." http://www.dmt-nexus.me/forum/default.aspx?g=posts&t=398
- ↑ Marsofold (@ The Nook Forums). 2005. "Extraction Of An Alternate Alkaloid From Mimosa." http://www.thenook.org/forum/index.php?showtopic=39278
- ↑ Doctorcito (@ Ayahuasca Forums). 2006. "Yuremamine: Solving the Mystery of Jurema Preta?" http://forums.ayahuasca.com/phpbb/viewtopic.php?t=9912
- ↑ DonPeyote (@ Drugs-Forum). 2006. "Jungle DMT?" http://www.drugs-forum.co.uk/forum/showthread.php?t=26468
- ↑ Noman (@ DMT-Nexus Forums). 2006. "Dark DMT - the Other Alkaloid." http://www.dmt-nexus.me/forum/Default.aspx?g=posts&t=90
- ↑ Noman (@ The Lycaeum Forums). 2006. "Dark DMT." http://forums.lycaeum.org/index.php/topic,17215.0.html
- ↑ Vepsäläinen JJ, Auriola S, Tukiainen M, Ropponen N, Callaway JC. 2005. "Isolation and characterization of yuremamine, a new phytoindole." Planta Med. 71(11):1053-7
- ↑ Marsofold (@ Drugs-Forum). 2005. "The Other Alkaloid In Mimosa Hostilis." http://www.drugs-forum.co.uk/forum/showthread.php?t=13507
- ↑ Noman (@ DMT-Nexus Forums). 2006. "Dark DMT - the Other Alkaloid." http://www.dmt-nexus.me/forum/Default.aspx?g=posts&t=90
- ↑ Vepsäläinen JJ, Auriola S, Tukiainen M, Ropponen N, Callaway JC. 2005. "Isolation and characterization of yuremamine, a new phytoindole." Planta Med. 71(11):1053-7
- ↑ Noman (@ DMT-Nexus Forums). 2006. "Dark DMT - the Other Alkaloid." http://www.dmt-nexus.me/forum/Default.aspx?g=posts&t=90
Analogues
<onlyinclude>
Brief overview - What is 5-MeO-DMT?
5-MeO-DMT is a potent naturally occuring psychedelic alkaloid
Chemical and physical properties
For solubility, melting point, etc, check the 5-MeO-DMT Chemical and Physical Properties WIKI
5-MeO-DMT can be oxidized naturally or by the use of hydrogen peroxide and becomes 5-MeO-DMT N-Oxide
Effects
Excessively fast onset - 10-30 seconds.
Lower doses (2-6 mgs smoked) - Crushing body load quickly yielding a sense of body-orgasm. No CEV's, slight fractal visual distortions with open eyes. Peak effects 5-25 mins. Total time to full baseline 1.5-2.0 hours
Medium doses (7-15 mgs smoked) - Crushing body load yielding gasping and gagging nausea with any resistance and a sort of release into the 5-MeO state with surrender - beyond body-orgasm into gasping soul orgasm. CEV's show a sort of yellow or white field. Eyes want to open and close. Open eye visuals are best with living, loved ones. Fractalized in space and time. Peak effects 20-40 mins. Total time to full baseline 1.5-2.0 hours.
Higher doses (16-22 mgs smoked) - Body load that hits like a wall of drowning whiteness. Overwhelming. No time to resist or submit or feel nauseaus or groan or . . . Blackout for peak memories. Weird verbal outbursts or babbling. Extended peak effects, 20 mins to over an hour. Total time to baseline 2 hours to . . . . extended feeling of not being fully back or integrated that can last days, weeks or months.
It is worth noting that different anecdotal reports of 5-MeO-DMT use differ significantly in terms of their descriptions of the effects that particular dosages bring on. While some people have reported that 30 mgs produces only threshold effects, others have reported being completely overtaken by as little as 5 mgs. These drastic variations could be due to physical differences between individuals or variations in substance purity.
In order to maximize safety, it is recommended that potential users of 5-MeO-DMT start dosing themselves at the low end of the spectrum (2-6 mgs) before attempting higher dosages. As discussed earlier, even low doses of 5-MeO-DMT can occasionally produce extremely overwhelming effects.
Pharmacology, toxicity and general safety
Full agonist at 5-HT 1A, 5-HT 1c, 5-HT 1d & 5-HT 2, Callaway & McKenna 1998 5-HT receptor interactions & specificities: McKenna et at. 1990 Sec also: Glennon et al. 1979 & 1994; Sanders & Bush 1967
Distribution, mctabolism & excretion (in rat): Sanders & Bush 1967 Preferentially metabolized by MAO-A:Squires 1975; Suzuki et al. 1981
When ingested together with a MAOI, 5-MeO-DMT is O-demethylated by CYP2D6 and becomes Bufotenine in the liver, but without a MAOI, it is mostly N-Demethylated by MAOI into 5-MeO-Indole-acetic-acid (Shen et al 2010; Ai-Ming 2008).
There is some concern regarding taking 5-MeO-DMT orally, specially with a MAOI. There has been at least one reported death (Sklerov, 2005; Callaway 2006), and some hospitalizations. This might be due to individual metabolic differences. Check this thread for more info. If you are consuming 5-MeO-DMT orally for the first time, specially with MAOIs, start VERY low and raise your dosage gradually.
General For info on DMT safety, please reffer to Health and Safety tips regarding set and setting, how to integrate and so on, as written for DMT, are also relevant to 5-MeO-DMT
Life-forms containing 5-MeO-DMT
Acacia spp.
The alkaloid information about Acacia coming from TLC analysis is not to be considered definite. They are tentative identifications that need to be confirmed with GC-MS or other appropriate analytical methods. (ref Trout's Notes)
- Traces 5-MeO-DMT tentatively observed in twigs. 5 Oct. 1995. TLC by J. Appleseed 1995 (Xanthydrol), co-occuring with suspected DMT. (Trout's Notes)
Acacia angustissima Trace amounts tentatively observed in roots (unconfirmed) in March 1995. TLC by J. Appleseed. Not ob- served in second assay. Trace amounts in seeds. tlc by Appleseed 1995 (all with Xanthydrol)
Acacia auriculiformis Trace amounts tentative ly ()bserved in stem-bark (25 April 1995). tic by J. Appleseed 1995 (A band at this Rf was also seen in roots [mislabeled as Guaiacuml in 2 Sept. 1994 assay but used Ehrlichs reagent which does not differentiate between DMT and 5-MeO-DMT.)
Acacia cultriformis Blue band co-chromatographing with 5-MeO-DMT (Xanthydrol) Seen in branch stems, and in phyllodes, also in flowering spikes. Commercial florist's material. Sept 1996.
Blue band co-chromatograpbing with 5-MeO-DMT (Xanthydrol) Roots of two year old seedlings. TLC by J. Appleseed 1996. Also in stems Sept 1996. Not observed in roots Sept. 1996.
Acacia farnesiana Traces tentatively observed in green fruit. Not present in ripe fruit. (Xanthydrol) Also contained a suspected beta-carboline. (blue under UV) 24 July 1995. tlc by J. Appleseed 1995.
Acacia maidenii Traces observed in wood (October 1995); Observed in twigs (26 July in phyllode and in mixed phyllode and twigs 27 Oct 1995) tic by J. Appleseed 1995 (Xanthydrol) Co-occurring with suspected DMT in all samples of phyllodes and twigs but sole alkaloid seen when just using twigs. Not observed in bark or root bark. Note: Acacias such as this one do not produce leaves except when young or stressed. What are thought of as being leaves are actually phyllodes; a specially modified petiole (the short stalk at the leaf base.
Acacia nilorica
Faint traces observed in stem, roots and leaves. As-
sayed separately (Sept 1996; Xanthydrol)
Acacia obtusifolia
In some samp les as a minor component. Apparently
unpublished work. Pers. comm. with Snu Voogelbreinder. Needs confimation.
Appeared present in one sample of leaves / twigs looked
at by Mulga: Not observed in bark or root bark.
Acacia victoriae Strong blue band eo-chromatographing with 5-McO- DMT (Xanthydrol) Roots of two year old seedlings. tic by J. Appleseed 1996.
Anadenanthera spp.
Anadenanthera spp. (G. Seitz, 1965]
- Seedlings 0.001% 5-MeO-DMT [4% of 29 mg of total alkaloic / 100 gm dry.) (Schultes et al 1977 ref Trout's Notes)
- (As Piptadenia sp.) Caspar, 1964; Guapore, Brazil; Tupari. - Seeds: 0.11% 5-MeO-DMT (85% of 13 mg of total alkaloids / 100 grm) (Schultes et al 1977 ref Trout's Notes)
- 5-MeO-DMT reported in snuff thought to originate from this species. Conflicting reports. Most accounts have found only bufotenine in the seeds but several reports exist claiming the presence of DMT and/or 5-MeO-DMT. Torres et al. 1991 reported the detection of all three in snuff powder recovered from archaeological sites in Argentina believed to have been derived from A. colubrina seeds. Both A. colubrina and A. colubrina var. cebil occur in Argentina. While it is not clear which Torres and coworkers referred to; the latter is implied. No analysis of seeds or verifiable plant materials were reported in Torres et al. 1991.
- No.24625; Origin: Boa Vista, Brazil - Bark- 0.025% (25mg of 5-MeO-DMT / 100 gm. of dry bark]
Leaf- 0.006% [6 mg. of 5-MeO-DMT / 100 gm. of dry leaves] (Agurell et al. 1969 ref Trout's Notes)
- (as Piptadenia peregrina) Legler & Tschesche 1963 examined bark and reported 5-MeO-DMT formed 32% of the crude base. A mixture of 5-MeO-NMT and NMT comprised 41%. Used Brazilian material. Seeds collected in western Brazil in the Rio Branco region during 1953 5-MeO-DMT ; Bark collected in Colombia during 1956 with 5-MeO-DMT (Holmstedt & Lindgren 1967 ref Trout's Notes)
- No number; La Carolina, Barrio St. Just, near San Juan, Puerto Rico, December 1974. Same colony as Schultes 26363. - Mature seeds collected in March 1975; hill behind El Comandan te horse-racing track. 1975 analysis (5 months after collection): 5-MeO-DMT- 1% of total alkaloid. 1977 analysis of same material could detect only bufotenine (80% of total alkaloid in 1975). (Schultes et al 1977 ref Trout's Notes)
- R. E. Schultes 26363; La Carolina, Barrio St. Just, near San Juan, Puerto Rico, Dec. 1972 - Immature seeds collected December 1972 0.04% 5-MeO-DMT (19% of 209 mg of total alkaloid/ 100 gm dry)
Seedlings 0.024% 5-MeO-DMT (95% of25 mg of total alkaloids / 100 gm dry] Pods without seeds 0.012% 5-MeO-DMT (91% of 13 mg of total alkaloids / 100 gm dry] Leaves 0.094% 5-MeO-DMT (88% of 107mg oftotal alkaloids / 100 gm dry] Twigs 0.0357% 5-MeO-DMT (94% of38 mg of total alkaloids / 100 gm dry) Bark (0.41% total alkaloid) 0.39% 5-MeO-DMT (95% of 410 mg of total alkaloids / 100 gm dry) Roots (0.69% total alkaloid) 0.678% 5-MeO-DMT (97% of 699 mg of total alkaloid / 100 gm dry] (Schultes et al. 1977 ref Trout's notes)
- R.E.Schultes 24625; Boa Vista, Brazil)
Leaves 0.00624% 5-MeO-DMT (48% of 13 mg of total alkaloids / 100 gm dry] Bark 0.025% 5-MeO-DMT (59% of 42 mg of total alkaloids / 100 gm dry) (Schultes et al. 1977 ref Trout's notes)
- Snuff: "epena"
Obtained from the Waica by George Seitz. 5-MeO-DMT was the major alkaloid. Bufotenine was present as a minor component. Because of this, the claimed plant source (Virola) has been questioned. Holmstedt 1965 Many snuff using people use both snuffs, although many have preferences one way or the other. While idle speculation; we wonder if preparation might not have involved both plants, or if the snuff witnessed as made from one source ( Virola) may not have been contaminated with residuals of ano ther snuff (Anadenanthera) during processing or storage, in- volved the admixture of other Myristicaeaous plants or as-yet unidentified plants, or perhaps have been derived from an ·altogether different but as yet un- known source.
- Snuff: "parica"
Snuff as prepared by Piaroa Indians (collected 1955) 5-MeO-DMT [with DMT and Bufotenine] Holmstedt & Lindgren 1967
- Snuff: "yopo"
Snuff prepared, by the Pix.asi-teri (or Bisashi-teri) of Upper Orinoco, from the seeds of an Anaden.anthera species. 5-MeO-DMT [with Bufotenine) Marini-Bettolo eta/. 1964
- Snuff: "yopo"
Snuff collected in Colombia (collected 1956) 5-MeO-DMT [with DMT and Bufotenine] Holmstedt & Lindgren 1967
Arundo spp.
Arundo donax Leaf and flower. (Ghosal 1972a ref Trout's Notes)
Bromus spp.
(Brome grass)
5-MeO-DMT appeared potentially present in at least some specimens of one Bromus sp. (B. breviaristatus) 1996 tlc by J. Appleseed. Species was grown by Giorgio Samorini from seeds provided by Trout and identified at seed maturity by Dr. F. Festi in 1999. (Ref Trout's Notes)
Caesalpinia spp.
- In stem-bark (with 1 other band) 1996 assays.
- In roots. (Nice band with two others present) June
1995, also 1996. tlc by J. Appleseed (Xanthydrol) (Ref. Trout's Notes)
- In flowers and buds. (26 August 1995) tlc by J.
Appleseed 1995. [August 1994 of a small sample of dead flower petals did not detect this alkaloid but did show a faint indolic band at a lower Rf.] In roots (nice band) June 1995, also 1996. tlc by J. Appleseed 1995 (Xanthydrol) Desmodium sp. (Wild species, not yet positively iden- tified; Austin, Tx.) Trace amounts in aerial portions (co-occurring with suspected DMT). tlc with Xanthydro1 spray, 24 June 1995. (Ref. Trout's Notes)
Delosperma spp.
TLC assays by Johnny Appleseed (ref Trout's Notes)
- May 1995 assay, 5-MeO-DMT Faint (Xanthydrol) Nov. 1995 assay: Dark blue and purple band corresponding to DMT and 5-MeO-DMT. (Xanthydrol) Other earlier positives used Ehrlich's.
- 2 Nov. 1995 sample. 5-MeO-DMT, Very nice dark band with Xanthydrol.
Delosperma cooperi May 1995 assay (two sources) also in 2 Nov. 1995 sample. 3 positives total with Xanthydrol. Other positives using Ehrlichs; co-occurrence with DMT seen in 2 Nov. 1995 sample.) (Sasha did not confirm on material from another source.
- 5-MeO-DMT Nice single blue band. Xanthydrol. Sept. 1996.
- 5-MeO-DMT. 2 Nov. 1995 sample. Dark band with Xanthydrol.
Delosperma harazianun Audhali Plateau, Yemen
- 5-MeO-DMT. 2 Nov. 1995 sampling. Dark band with Xanthydrol.(Co-occurring with DMT) Not observed In D. harazianum Shibam.)
- Nice 5-MeO-DMT band.· Xanthydrol. Sept. 1996. (Co-occurrence with DMT)
- 2 Nov. 1995 Sample. 5-MeO-DMT. Dark band with Xanthydrol.
- 9 May 1995. Weak 5-MeO band. (Xanthydrol)
- (Same individual plant tested Dec. 1994)
Positives: May and 2 Nov. 1995. [Faint in Nov. Good in May] (Xanthydrol.). Material harvested Aug. and Dec. 1995 tested positive (with DMT co-occurrence); Sept. 96 (also using Xanthydrol). Assay with Ehrlich's had shown decent band at this Rf in 5 Dec. 1994 sampling.
Desmodium spp.
- Aerial parts (0.057% by wet weight; 0.57 gm. from 1kg. of fresh wet material.) Banerjee & Ghosal 1969 (Ref. Trout's Notes)
Stem/leaf (Ghosal 1972a Ref. Trout's Notes)
- Green Plant (Stem and Leaf) Ghosal & Bhattachaya 1972 (Ref. Trout's Notes)
- Our assays of seedgrown plants detected 5-MeO-DMT as present in trace amounts in seeds
(May 1995 assay). No alkaloids were observed iu this species until after it was 2 years old. 5-MeO-DMT was observed in small amounts in roots and also in stems (May 1995); Also in leaf Feb. 1995 (using large red leaves left from winter) and May 1995 using normally colored new but full sized leaves. Leaves also tested positive (faint) in Nov. 1995. tlc by J. Appleseed using Xanthydrol. (Ref. Trout's Notes)
- Leaves (35+ mg. from 2 kg) Ghosal e1 al 1972a (Ref. Trout's Notes)
ln stem/leaves Ghosal 1972a (Ref. Trout's Notes) Roots Gihosal 1972a (Ref. Trout's Notes)
Desmodium pulchellum Bentham ex Baker
- Whole plant (0.2-0.25% by dry weight.) Ghosal &
Mukherjee 1964 (Major alkaloid. Ghosal & Mukherjee 1965. (Amount not given. Plates crystallized from 8.36 grams of impure chromatographic fraction resi- due; from 4 kg of dried whole plant.) Ghosal & Mukherjee 1966 (Ref. Trout's Notes) Stem and leaf of young seedl ing [Trace.] Ghosal et at. 1972c Stem and leaf of mature plant [0.476% by dry weight; 34% of 1.4% Total alkaloid] Ghosal eta!. 1972c Root of mature plant [0.132% by dry weight; J 2% of 1.1% Total alkaloid] (Also, in same paper; 1.8 kg. of dried roots yielded 0.23 gm; i.e. - 0.013% by dry weight.) Ghosal et a/. 1972c Seeds (ripe) of mature plant [0.002% by dry weight; 10% of0.02% Total alkaloidJ Ghosal eta/. 1972c Root, stem-leaf and flower (A mounts not given) Ghosal 1972a
- Whole plant. (Amount not given) Hsli et a/. 1982
cited HsU 1970 [Source article has not been located. Title is suspect.]
Dictyoloma spp.
Dictyoloma incanescens· DC 0.04% isolated from dry bark collected in winter near Rio de Janeiro. Voucher prepared. (Pachter et al 1959 ref Trout's Notes)
Dutaillyea spp.
Dutaillyea drupacea (Baillon) Hartley New Caledonia
- 5-MeO-DMT was the sole alkaloid (98%) in the leaves- 0.04%. [The published value of 0.4%, repeatedly appearing in the literature, IS A TYPO] (Baudouin et al. 1981 ref Trout's Notes)
- 5-MeO-DMT was absent from trunk-bark (Baudouin et al. 1981 ref Trout's Notes)
Dutaillyea oreophila (Baillon) Sevenet-Pusset New Caledonia
- Leaves- 0.02% 5-MeO-DMT by dry weight. Major alkaloid (40% of 0.05% total alkaloids) Co-occurring with 2-Methyl-6-methoxy-tetrahydro-beta-carboline (0.005% dry wt), Hordenine (0.0125% dry wt.) and Kokusagine (0.0125% dry wt.) (Baudouin et al. 1981 ref Trout's Notes)
- 5-MeO-DMT was absent from trunk-bark (Baudouin et al. 1981 ref Trout's Notes)
Digitaria spp.
(Crab grass)
Digitaria sanguinalis 5-MeO-DMT appeared potentially present in at least one local species; probably D. sanguinalis. Positive identification pending. 1996 tlc by J. Appleseed. (Ref Trout's Notes)
Diplopterys spp.
- Not found by Mckenna 1984, who only found DMT and traces of bufotenine
- Not found by Endlessness who only found major DMT peak (small peaks in process of identification but are NOT 5-MeO-DMT).
- 0.4655% DMT, co-occuring with traces of NMT, Bufotenine and MTHBC (Agurell et al. 1968) ( misidentified as Banisteriopsis rusbyana see Gates 1982)
- 0.16638% DMT, 0.0035% MTHBC and 0.0035% 5-MeO-DMT in dried stem (Agurell et al. 1968) ( misidentified as Banisteriopsis rusbyana see Gates 1982)
Evodia spp.
- 5-MeO-DMT in unripe fruit. Takagi et al. 1979 recovered 30 mg from 10 kg unripe fruit. Yu et al. 1997 reported 0.00015% by dry weight in the unripe fruit [ 15 mg from 3 kg.l
- Also present in the roots. Shulgin & Shulgin 1997
- 5-MeO-DMT (0.21% dry wt.) is the major alkaloid (35% of 0.61% total alkaloids) in aerial parts. Accom panied by 0.03% 5-MeO-DMT-N-oxide and 0.024% 2-Methyl-6-methoxy-tetrahydro-beta-carboline, Acronydine (0.61%), Kokusaginine (0.183%), Acronycidine (0.0 12%), Melicopicine (0.043%), Melicopidine, 0.018%) and Acronycine (0.006%). (Skaltsounis et al. 1983 ref Trout's Notes)
Horsfieldiana spp.
Horsfieldiana superba (Hk. f. ctTh.) Warb.
- Collected in September at Sandakan, Sabah, Malaysia.)
5-MeO-DMT as minor leaf alkaloid (0.0007%; 20mg from 2.8 kg of lea ves.] Roots and bark apparently unexamined. (Jossang et al 1991 ref Trout's Notes)
Hugonia spp.
Hugonia oreogena Schleeter
- Traces of 5-MeO-DMT in rootbark. (0.5 mg from 140 grams) (Ikhiri et al. 1987 ref Trout's Notes)
Iryanthera spp.
- Trace amounts in bark Holmstedt et al. 1980
Justicia spp.
- In leaf. Shulgin & Shulgin 1997
- var. stenophylla -
Leaf (dry) sampled 2 Nov. 1995 showed a faint blue band that co-chromatographed with 5-MeO-DMT. Plant from LER. TLC by Applesecd 1995. (Xanthydrol) We wonder if the above might not actually be the same alkaloid as McKenna observed rather than 5-MeO-DMT. While TLC has indicated DMT traces in this SAME plant, this was our only sampling to show this compound and no DMT.
Lespedeza spp.
Lespedeza bicolor Turcaninow var japonica Nakai
- In leaf and root bark. Smith cited Goto et al 1958 (Ref Trout's Notes)
- Present in root bark (Less than 0.1%). Morimoto & Matsumoto 1966 (Ref Trout's Notes)
Lespedeza bicolor Our assays did not detect any alkaloids in any parts during the first year. ( 1994) We did detect 5-MeO-DMT in both seeds and mixed seeds and pods. (May 1995) tlc by J Appleseed. See comments under DMT as we may have mistaken the two as tlc used Eh.rlichs (Ref Trout's Notes)
Mimosa spp.
- 5-MeO-DMT in low amounts in stem and leaf after the first year
(November harvest of 15 month old plants). Concentrations were higher in the roots (August harvest). Assays 2 Nov. 1995 Very young seedlings (whole plant) tested in 1996 showed a very dark suspected 5-MeO-DMT band.
- In branches; 1996 assays.
tlc by J. Appleseed 1995-6 (ref trout's Notes) [DMT did not start to show up in assays until after second year, at which time it was present in leaf and root.]
- Maybe appearing in error? Not observed by Gupta and coworkers
Mimosa tenuiflora (Willd.) Poir. The listing of 5-MeO-DMT is in error. Meckes-Lozoya ran it as a pure reference sample only.
Mucuna spp.
- 0.01% in fresh leaves Ghosal et al 1971
- In leaf seed, stem and roots. Ott cited Bhattacharya et al 1971; Ghosal 1972a; Ghosal et al. 1971
- In root, stem-leaf and pod Ghosal 1972a
Osteophloem spp.
Osteophloem platyspermum (DC) Warb. (Schultes &Rodrigues #26126]
- One of 3 alkaloids in 0.62 mg of total alkaloid from 100 grams of dry bark. (Brazil);
- Other material: [Schultes & Tovar #7095 (Peru)] tested negative with Ehrlich's and Dragendorff's. (Holmstedt et al. 1980)
- McKenna et al 1984b tested a samples of leaves DMK-78 but failed to find. Did report Methyltryptophan methyl ester
Other Grasses
??? spp. (Wild Rye, Winter Rye, Rye Grass) 5-MeO-DMT appeared to be potentially present in several local species (2-4 spp.; including both annual & perennial ryes. Including the haying material called "coastal') 1996 tlc by J. Appleseed. (Ref Trout's Notes)
Phalaris spp.
Phalaris data below is incomplete. Alkaloid concentrations and proportions are highly variable from year to year and show dramatic seasonal fluctuations. Concentrations between plant parts and first growth versus regrowih are also very different. In many populations there may be marked differences in both the amounts present and in actual alkaloid profile from one plant to the next. (i.e. plants in the same population and arising from the same seeds may show completely different chemistry, not simply differing concentrations.) See the amazing Festi & Samorini pieces listed in the Phalaris spp. page about cultivation for a review and overview of what is known so far.
- A major alkaloid in all samples they examined. (Culvenor et al. 1964)
- 5-MeO-DMT was not present in all clones examined (4 out of 12) Frahn & Illman 1973
- 5-MeO-DMT in leaf. 0.01-0.28% in material from California. (Festi & Samorini 1994a cited Welch 1971 ref Trout's Notes)
- var. AQ-1 (Italy) Weak occurrence reported (HPLC). (Festi & Samorini 1994b ref Trout's Notes)
- as Phalaris tuberosa - Strong in leaf. 2 Nov. and 17 Sept. 1995. Dec 1995. Assay. tlc by J. Appleseed 1995 (ref Trout's Notes)
- var Killer Phalaris (At one point this was synonymous with cv. Uneta, but has been in uncontrolled propagation for long enough this is no longer certain.) 5-MeO-DMT predominated in 25 June, 17 Sept., 2 Nov. 1995 samples. (DMT was predominate alkaloid in Fall 1994 by TLC) (ref Trout's Notes)
- Clone #R5 "large" amount of DMT (5-MeO? possible mistake in Trout's Notes) co-occurring with twice amount of 2-MTHBC (Clone, designated 405-9, originating with U.S. Regional Pasture Research Laboratory, University Park, Pennsylvania)
- Clone #R37 ·'trace" amount of 5-MeO-DMT co-occurring with " intermediate" amounts of hordenine and "large" amounts of 6-methoxy-2,9-dimethyl-1,2,3,4-
tetrahydro-beta-carboline. (From highly diverse source population used in plant breeding and genetic studies at the University of Minnesota. Department of Agronomy and Plant Generics".)
- Clone #R51 ·'large" amount of 5-MeO-DMT as sole observed al.kaloid. [Same source as R37)
- Clone #R96 " large" amount of 5-MeO-DMT co-occurring with "trace" amounts of hordenine and "trace" amounts of 6-methoxy-2,9-dimethyl-1,2,3,4-tetrahydro-beta-carboline. (Same source as R37) (Gander et al. 1976. ref Trout's Notes)
- cv. Australian Commercial (CPI 119305) A major alkaloid in 7 day old seedlings. 150 nmol / 100 seedlings. (Mulvena & Slaytor 1983 ref Trout's Notes); In seedlings. (Mack et al. 1988); Mature 0.05% dry wt.(Baxter & Slaytor 1972)
- cv. Sirocco 51 nmol / 100 seedlings. (Mulvena & Slaytor 1983 ref Trout's Notes); Major base. (Frahn & O'Keefe 1971); 5-MeO-DMT in leaf (Ott 1994 cited Culvenor et al 1964; Baxter & Slaytor 1972; frahn & Illman 1973 ; Moore et al 1967; Mulvena & Slaytor 1982; Oram & Williams 1967 ref Trout's Notes)
- 5-MeO-DMT in leaf and whole plant Barnes et al 1971; Culvenor et al 1964; Gander et al. 1976; Majak & Bose 1977; Majak et a!. 1978; Marten et al. 1973
Williams et al. 197 1. Many others.
- 0.0002-0.0067% 5-MeO-DMT in material from British Columbia. (Majak & Bose 1977)
- 0-0.02% in material from Minnesota. NRG741 was strongest of those tested & NRG721 the weakest. (Majak eta/. 1978)
Festi & Samorini 1994a
- tlc by Johnny Appleseed
- P.I. 172442 Turkey (cv. Turkey Red)0.0025% to 0.045% total alkaloid by wet weight. 5-MeO is predominate alkaloid. (J.Appleseed (undated manuscript); "Ayahuasca analog plants of the temperate zone." Tlc by Johnny Appleseed: fall 1994, 25 June, 17 Sept, 2 Nov. 1995. (ref Trout's Notes)
- cv Ottawa Synthetic : 5-MeO-DMT present, Amounts not given. Detected by TLC (Woods & Clark 1971 ref Trout's Notes)
- PI 202676, PI 231044: 5-MeO-DMT reported, Appleseed tlc evaluation of field trials using USDA seeds. (ref Trout's Notes)
- PI 167261, and also 284185 (lower levels) 5-MeO-DMT reported, Appleseed tlc evaluation of field trials using USDA seeds. (ref Trout's Notes)
- Traces reported (HPLC). Festi & Samorini 1994b
Phalaris stenoptera (= P tuberosa var. stenoptera)
- Variable amounts. Festi & Samorini 1994b cited Rendig et al 1970 as finding 135-264 J.Lg/ml of expressed juice. (ref Trout's Notes)
Sorghum spp.
Sorghum halepense (Johnson Grass, Aleppo Grass, Egyptian Millet, Grass Sorghum, Means Grass)
- Low levels of 5-Me0-DMT in leaf only. 1996 TLC by J. Appleseed. (Xanthydrol) Samples collected spring and summer; Central Texas. (ref Trout's Notes)
Umbellularia spp.
Umbellularia californica (Hook & Am.) Nutt.
- Unspecified concentration of 5-MeO-DMT in stembark. Needs confirmation. (Ratsch 1998 ref Trout's Notes)
Virola spp.
Snuff and other products from unidentified Virola.
- Virola based arrow poison (Yanomamo). 8% 5-MeO-DMT by dry wt. (Macrae & Towers 1984 cited Galeffi et al 1983 ref Trout's Notes)
- "epena" - Snuff as prepared by Araraibo Indians (collected 1965) - 5-MeO-DMT [with DMT]
- Snuff as prepared by Tucano Indians (collected 1965) - 5-MeO-DMT [with DMT and 5-MeO-MMT)
- Snuff as prepared by Waica Indians (collected 1965) - 5-MeO-DMT [with NMT and DMT] (Holmstedt & Lindgren 1967 ref Trout's Notes
- epena" [N.24574; Origin: Rio Cauaburi, Brazil) - 0.5% 5-MeO-DMT [515 mg. of 5-MeO-DMT / 100g snuff) (Agurell et al. 1969 ref Trout's Notes)
- nyakwana (n24626; Origin: Tototobi, Brazil] - 9.68% 5-MeO-DMT [9,6130 mg. of 5-MeO-DMT / 100g snuff) (Agurell et al. 1969 ref Trout's Notes)
- Suspected virola snuff (Based on chemistry, not voucher) - 1.4% 5-MeO-DMT. (De Budowski et al. 1974 ref Trout's Notes)
- Virola paste (No voucher; " oo'-koey" ; La Chorrera) - 5-MeO-DMT 1.19 mg/ ml. DMT also present at O.3 mg/ ml (Mckenna et al 1984a ref Trout's Notes)
- (Collected on March 12; Santa Rose, Iquitos, on Rio Momon in Peru) Wood was found to contain 5-MeO-DMT (major alkaloid) co-occurring with 2-Methvl-6-methoxy-1,2,3,4-tetrahydro-Beta-carboline and nicotine. No amounts given. Miles et al 1987 ref Trout's Notes
- Plowman, Schultes and Tovar #7093; Origin: Pebas, Peru.) (5-MeO ?) DMT in bark Holmstedt et al. 1980 ref Trout's Notes
- 5-MeO-DMT has NOT been found on all samples
- bark 0.245 mg of 5-Me0-DMT/ gm dry bark. Macrae & towers 1984 cited McKenna et al. 1984a
- Plowman, Schultes and Tovar #6920; Origin: Pebas, Peru. Present in resin and phloem (Not in leaves) Holmstedt et al. 1980 ref Trout's Notes
- Plowman, Schultes and Tovar #7263; Origin: Pebas, Peru. Present in paste and phloem. Holmstedt et al. 1980 ref Trout's Notes
- Plowman, Schultes and Tovar #7092; Origin: Pebas, Peru. Trace in bark. Holmstedt et al. 1980 ref Trout's Notes
- DMK-59. 5-MeO-DMT present in bark (not in leaves- DMT and NMT present in leaves). McKenna et al. 1984b
- DMK-67, DMK-68 and DMK-69. 5-MeO-DMT in leaf (Negative assay in bark) McKenna et al. 1984b
- Paste - DMK-59; Alfredo Moreno no. I. 5-MeO-DMT 2.03 mg/ ml (Sole base present). McKenna et al. 1984a ref Trout's Notes
- Many collections of this species were reported devoid of alkaloid. Others contained only traces of DMT.
- [No.24614; Origin : Manaus, Brazil) - Roots-0.00059% [0.59 mg. of 5-Me0-DMT/ 100 gr dry wt) Agurell et al 1969 ref Trout's Notes.
- (Schultes No. 24616; Origin Manaus, Brazil] - Trace amounts of 5-MeO-DMT in dry roots. 590ug per 100 grams. Holmstedt et al. 1980 ref Trout's Notes
- 5-MeO-DMT In bark and paste. Holmstedt et al. 1980 ref Trout's Notes
- 5-MeO-DMT in plant. Part and amount not given. In roots (even lower levels). Lai et al. 1973 ref Trout's Notes
- [Schultes N.24612; Origin: Manaus, Brazil). Bark- 0.008%? [8 mg. of 5-MeO-DMT per 100g bark) given in Holmstedt et al. (1980) as 190 mg per 100 grams of dry bark which, if true, would be 0.19%.)
- Roots- 0.135% [ 135 mg. of 5-MeO-DMT per 100g root) - Agurell et al 1969
- Sample DMK-40. Found 5-MeO-DMT in bark. McKenna et al. 1984b
- Schultes No. 24595; Origin: Manaus, Brazil : * Bark- 0.11% [108 mg. of 5-MeO-DMT/ 100 gm. ) * Roots- 0.011% [ 11 mg. of 5-methoxy-DMT/ 100 gm.]
- Leaves - Traces. Agurell et al. 1969
- Schultes No. 24626; Origin: Tototobi, Brazil] * Bark- 0.062% [62 mg. of 5-Me0-DMT / 100 gm.) Agurell et al. 1969 ref Trout's Notes. Ott 1993 cited Holmstedt 1965
- Schultes No. 24613; Origin: Manaus, Brazil. * Roots- 0.001% [1 mg per 100 gm.]* Leaves- <0.001% [Less than 1 mg per 100 grams. (Holmstedt at al. 1980 listed only DMT in leaves for this same collection)]
- No. 24613; Origin: Manaus, Brazil. * Roots- 0.001% [1 mg per 100 gm.]* Leaves- <0.001% (Less than 1 mg per 100 grams. (Holmstedt et a. 1980 listed only DMT in leaves for this same collection) Agurell et al. 1969 ref Trout's Notes (COPY OF ABOVE ANALYSIS FROM V. THEIODORA? MISTAKE?)
- Plowman, Schultes and Tovar N7094; Origin: Pebas, Peru): * Bark- 0.0042% [4.1mg per 100 grams of dry roots. * Leaf- 0.0003% [0.29 mg per 100 grams of dry leaves] Holmstedt et at. 1980 ref Trout's Notes
Humans and other animals
- Cutaneous glands were found to contain as much as 60 to
160 mg per gram of dry tissue 1.0-3.5 mg/ gm of non-glandular skin. (Erspamer et al. 1965 ref Trout's Notes)
- As much as 5- 15% of parotid and coxal glands by dry
weight. Ranged from 50-1 50 mg per gram oflarge cutaneous glands (dry) and from 0.42-3.5 mg per gram in the rest of the dry skin. (Erspamer et at. 1967 ref Trout's Notes (occurs with its N-sulfate)
- Sample analysis of human cerebrospinal fluid included 5-MeO-DMT. (Christian et al. 1975 ref Trout's Notes)
- Found in cerebrospinal fluid of some psychotics and a few normal people by Corbett et al. 1978. Narasimhachari et
al. 1971b reported it was more common in psychotics than normals (ref Trout's Notes)
- Also detected in some patients by Smythies et al. 1979 but it is not clear in their account exactly which subjects showed its presence and which did not. (ref Trout's Notes)
Extraction teks
Any typical extraction teks for DMT should potentially extract 5-MeO-DMT (but it will also extract DMT together). For separating 5-MeO-DMT from DMT, still no fail-proof method has been developed. Column chromatography will work using acetic acid as an eluent and silica as mobile phase. DMT will elute first (Rf 0.5), then 5-MeO-DMT (Rf 0.6) and bufotenine last (Rf 0.65) (De Bukowski 1974 ref Trout's Notes)
Dosages and consumption methods
5-MeO-DMT is around 5 times more potent than DMT by weight.
Smoked, dosage is around 5-15mg.
Oral, according to Jonathan Ott, 30mg are active without the need for MAOIs. Mixing with MAOIs, it will be 3 times stronger, so a dosage will be around 10mg. There is some concern about 5-MeO-DMT's oral ingestion safety, and it might be related with individual metabolism differences. Check this thread for more info. If you are consuming 5-MeO-DMT orally for the first time, specially with MAOIs, start VERY low and raise your dosage gradually.
Insufflated, 5-20mg (Erowid)
History of usage
Analysis of 5-MeO-DMT
Colorimetric
Reagent color results here
GC-MS
Retention time: 12.946 (System used)
Other info: 5-MeO-DMT: EI/MS (m/z, %): 218 (M+ , 10), 160 (6.3), 145 (2.5), 117 (5.0), 58 (100), 42 (4). (Takahashi 2008)
InfraRed
5-MeO-DMT freebase
NMR
1H NMR (400 MHz, D2O) δ ppm 7.47 (d, J=8.90 Hz, 1 H) 7.32 (s, 1 H) 7.20 (d, J=2.45 Hz, 1 H) 6.97 (dd, J=8.90, 2.45 Hz, 1 H) 3.91 (s, 3 H) 3.46 (t, J=7.43 Hz, 2 H) 3.20 (t, J=7.43 Hz, 2 H) 2.92 (s, 6 H).
(Source: Microgram Bulletin Vol. 3, Pg 8)
Other info: 1 H-NMR (CDCl , δ): 2.34 (6 H, s), 2.63 (2 H, m), 2.91 (2 H, m), 3.86 (3 H, s), 6.85 (1 H, dd, J = 2.3, 8.6 Hz), 6.98 (1 H, br d, J = 2.3 Hz), 7.05 (1 H, d, J = 2.3 Hz), 7.22 (1 H, d, J = 8.6 Hz). 13 C-NMR (CDCl3 , δ): 23.7, 45.4, 56.0, 60.1, 100.8, 111.8, 112.1, 113.9, 122.3, 127.8, 131.5, 153.9. (Takahashi 2008)
Scientific publications
Other links of interest
<onlyinclude>
Brief overview - What is Bufotenine?
Bufotenine (5-HO-DMT) is a psychoactive alkaloid found in several plants and animals, also in humans. It is known specially for it's presence in the skin and venom of some toads (Bufo species, hence the name BUFOtenine)
Chemical and physical properties
For solubility, melting point, etc, check the Bufotenine Chemical and Physical Properties WIKI
Bufotenine can be oxidized (naturally or with the use of hydrogen peroxide), and becomes Bufotenine N-Oxide
Effects
Pharmacology, toxicity and general safety
Jonathan Ott - Pharmañopo (bufotenine activity article)
Life forms containing bufotenine
Amanita spp.
(Generally low concentration. Ref Trout's Notes)
- Wieland & Motzel 1953 (European specimens) 0.045% bufotenine (Stijve 1979 noted their inefficient procedure may have only recovered 10% of what was there.)
- Bufotenine presence. Catafolmo & Tyler 1961 (North American specimens)
- Bufotenine presence. Tyler 1961 (North American specimens)
- Bufotenine presence.Tyler & Groger 1964 (Gennan specimens) Identified chromatographically. (Mycelium shown to contain 0.03%)
- Stijve 1979 (German, Dutch & Swiss specimens). Bufotenine was the major alk. Bufotenine concentrations were estimated from 0.70- 1.5% using GLC and from 0.4-1.3% using tlc. Isolation: 0.8% in cap. 1.5% iu stalk & 0.065% in bulb. Wurst et al. 1992 (0.0 - 1.899%)
- Bufotenine presence. Wieland & Motzel 1953 (observed)
- Catalfomo & Tyler 1961 could not verify
- Stijve 1979 could not detect it
- Wieland & Motzel 1953 (Bufotenine observed)
- Brady & Tyler 1959 could not detect.
- Stijve 1979 could not detect it
- Bufotenine presence. Catafolmo & Tyler 1961 (North American specimens)
- Bufotenine presence. Tyler 1961 (observed)
- Bufotenine presence. Tyler & Groger 1964 (German specimens) Identified chromatographically.
- Bufotenine presence. (0.374 & 0.617%) Wurst et al. 1992
- Bufotenine presence(0.018-0.020%). Wurst et al. 1992
- Stijve 1979 could not detect it
- Tyler 1961 (Bufotenine presence observed)
- Overview & summary: see Catalfomo & Eugster 1970
Anadenanthera spp.
Anadenanthera spp.
- Analyzing seeds, known to be used in preparing parica, collected in Brazil during the first half of the 1800s, DeSmet & Rivier 1987,reported finding only bufotenine. They thought that any other tryptamines may have degraded over time. They noted that Schultes et al. 1977, reported that 2 year old Anadenanthera seeds were observed to go through a similar change in composition while in storage and that Spruce's 1854 collection of A. peregrina seeds also yielded only bufotenine. [Identification of Anadenanthera species cannot reliably be made from seeds alone.] [It should be mentioned that Holmstedt & Lindgren 1967 detected both DMT and bufotenine in seeds that had been collected in 1948 and DMT and 5-MeO-DMT in seeds collected in 1956. Similarly Torres et al. 1991 detected DMT, 5-MeO-DMT and bufotenine in snuff dated to 780 AD]. Ref Trout's Notes
- "epena" Obtained from the Waica by George Seitz. Bufotenine was a minor component, Holmstedt 1965. Because of this, the claimed plant source (Virola) has been questioned. (Seitz claimed to have witnessed the preparation of the material from Virola. In light of the ambiguities uncovered in snuff analysis, we do not think this can automatically be considered to be an Anadenanthera snuff without further study. Our suspicion is that the botanical sources of snuffs remain incompletely characterized.) 5-MeO-DMT was the major alkaloid. (DMT also present as a minor)
- "epena" Snuff prepared, by Ma-hekodo-teri of the Rio Mavaca, from seeds of an Anadenanthera species. Bufotenine [with Bufotenine-N-oxide, DMT and
DMT-N-oxide) Marini-Bettolo et al. 1964 ref Trout's Notes
- "yopo" Snuff prepared, by Pixasi-teri (or Bisashi-teri) of Upper Orinoco, from an Anadenanthera species seeds Bufotenine [5-MeO-DMT also present] (Marini-Bettolo et al. 1964)
- "yopo" Snuff collected in Colombia (collected 1956) Bufotenine (DMT and 5-MeO-DMT also present] Holmstedt & Lindgren 1967 ref Trout's Notes
- "parica" Snuff prepared by Piaroa Indians (collected 1955) Bufotenine [DMT and 5-MeO-DMT also present) Holmstedt & Lindgren 1967 ref Trout's notes
- Snuff: "yopo" Believed to have originated from A . peregrina seeds; showed only bufotenine- 160 mg from 6 gm of snuff. (2.67%). De Budowski et al. 1974 ref Trout's Notes. Another Yopo sample showed only 5-MeO-DMT and was thought to be derived from a Virola species instead.
- Snuff: " epena". Bufotenine present. Yanomamo snuff prepared from Piptadenia peregrina. (Marini-Bettolo et al. 1964)
- 2.1% bufotenine yield from seeds collected in autumn near Rio de Janeiro. Voucher prepared. (Pachter et al. 1959)
- Conflicting reports. Most accounts found only bufotenine in the seeds but several reports exist of the additional presence of DMT and/or 5-MeO-DMT. Torres et al. 1991 reported the detection of all three in snuff powder recovered from archaeological sites in Argentina believed to have been derived from A. colubrina seeds. Both A. colubrina and A. colubrina var. cebil occur in Argentina. While it is not clear which Torres and coworkers referred to, the latter is implied. No analysis of seeds or verifiable plant material were reported in Torres et al 1991
- Modern unpublished analysis of seeds from Argentinean A. colubrina var. cebil found only bufotenine in material that proved active in humans used
as a snuff or when smoked. ref Trout's Notes
- var. cebil (= Piptadenia macrocarpa). Bufotenine Present in seeds. Not detected in pods: Fish et al. 1955. Material from both Florida and Brazil were used. ref Trout's Notes
- var. cebil In seeds and lower in seed pods. Iacobucci & Ruveda 1964. ref Trout's Notes
- var. cebil. Bufotenine 12.4% in seeds from a shaman's garden at Mision Wichi [Highest reported concentration of bufotenine in a
plant] , 4.41 % in a sample of seeds from Salta, 3.51% (seeds) and 0.05% (pods) in a second Salta collection. Only traces were found in bark from Cerro San Bernardo (All in Argentina). Torres & Repke 1996. ref Trout's Notes
Anadenanthera excelsa (as Piptadenia excelsa)
- In seeds. Lower concentration than P. macrocarpa. Iacobucci & Ruveda 1964
Anadenanthera falcata (as Piptadenia falcata)
- Major alkaloid in seeds. Giesbrecht 1960 ref Trout's Notes
- 1.1% bufotenine in seeds. Periera 1957 ref Trout's Notes
- Major alkaloid in seeds. (Haiti) Paris et al. 1967 ref Trout's Notes
- Present in seeds. Not detected in pods Fish et al. 1955. ref Trout's Notes. Material from both Puerto Rico and Brazil were used
- Present in seeds. Alvares Pereira & de Oliveira 1961 ref Trout's Notes
- Present in seeds Stromberg 1954 ref Trout's Notes
- R. Spruce # 119; Rio Negro, Brazil, collected in 1854) ( 1977 analysis). Seeds: 0.6% Bufotenine [614 mg/100 gm dry; Sole alkaloid. Schultes et al. 1977 ref Trout's Notes
- (as Piptadenia peregrina) Seeds collected in Puerto Rico during 1948 Bufotenine (DMT also present] Holmstedt & Lindgren 1967 ref Trout's Notes
- R.E.Scbultes, S.von R.Aitschul & B.Holmstedt, sin. num.; La Carolina, Barrio St. Just, near San Juan, Puerto Rico, December 1974. Same colony as Schultes 26363.]- Bufotenine in Mature seeds collected in March 1975; hill behind El Comandante horse racing track.
1975 analysis (5 months afler collection):No quantification - Bufotenine - 80% of total alkaloid; 1977 analysis of same material: 3.5% Bufotenine [3523 mg / 100 gm dry; Bufotenine was sole alkaloid present. Schultes et al 1977 ref Trout's Notes
- R. E. Schultes 26363: La Carolina, Barrio St. Just, near San Juan, Puerto Rico, Dec. 1972. In mature seeds collected December 1972: 0.01% Bufotenine [6% of 209 mg of total alkaloid/ 100 gram dry]; Seedlings 0.00025% Bufotenine [1 % of 25 mg of total alkaloid/ 100 gm dry]; Pods without seeds 0.00013% Bufoteni ne [1 % of 13 mg of total alkaloid / 100 gram dry]; Twigs 0.0004% Bufotenine 1 % of 38 mg of total alkaloids / 100 gm dry]; Roots [0.69% total alkaloid) 0.007% Bufotenine (1% of 699 mg of total alkaloid / 100 gm dry]. Schultes et al. 1977 ref Trout's notes
- Collected in southern Venezuela. Seeds- 7.5% bufotenine Schultes et al. 1977 cited Chagnon et al. 1970 & 1971 ref Trout's Notes
- [No.24625; Origin: Boa Vista, Brazil] Traces of bufotenine in dry bark. Agurell et al. 1969 ref Trout's notes
Arundo spp.
- Bufotenine In leaf and rhizome. 180 mg from 700 grams of rhizome. Ghosal et al. 1969 ref Trout's Notes
- Bufotenine 128 mg from 200 grams of dry plant. 110 plus 18 mg. Dutta & Ghosal 1967 ref Trout's Notes
- Bufotenine In flowers. Ghosal et at. 1971 ref Trout's Notes
Desmodium spp.
- Bufotenine major alkaloid in stem (0.04% by dry weight; If they used all of their picrate they would have recovered 4.3 gm of bufotenine base from 10.75 kg of stems.) Ueno et al. 1978 ref Trout's Notes
- Bufotenine in Leaf (68 mg. from 2 kg. of dry leaves.) Ghosal et al. 1972a ref Trout's Notes
- Bufotenine in Whole plant (Minor alkaloid) Ghosal & Mukherjee 1964; (Mention) Ghosal & Mukheijee 1965; (Amount not given) Ghosal & Mukherjee 1966 ref Trout's Notes
- Bufotenine in Stem and leaf of young seedling [0.011% by dry weight; 9% of 0.12% Total alkaloid] Ghosal et al. 1972c ref Trout's Notes
Lespedeza spp.
Lespedeza bicolor Turcaninow var japonica Nakai Present in both leaf and root bark. Morimoto & Matsumoto 1966 ref Trout's Notes
Mimosa spp.
- May be in error? Not observed by Gupta and coworkers ref Trout's Notes
Mucuna spp.
Mucuna pruriens In root, stem-leaf and pod. Ghosal (1972a ref Trout's Notes)
Osteophloem spp.
- Schultes & Rodriguez No. 26126; Origin: Manaos, Brazil. - Bufotenine in bark. One of 3 alkaloids in 0.62 mg of total alkaloid from 100 grams of dry bark. This is the only report of this compound being observed in a member of the Myristiacea. Holmstedt et al. 1980 ref Trout's Notes
- Plowman, Schultes & Tovar # 7095; Origin: Pebas. Peru (Alpha-Helix 1977) assayed negative with Dragendorff and Ehrlich reagents. ref Trout's Notes
Piptadenia spp.
- In seeds [with "related substances". Much lower concentrations than peregrina or Cebil. Von Reis Altschul 1964 page 7 cited a letter from M.S. Fish dated 7 January 1958. ref Trout's Notes
- In seeds (with "related substances" . Much lower concentrations than peregrina or Cebil. von Reis Altschul 1964 page 7 cited a letter from M.S. Fish dated 7 January 1958. Also by Yamasato et al. 1972 (TLC). Ref Trout's Notes
- In seeds [with "related substances". Much lower concentrations than peregrina or Cebil. von Reis Altschul 1964 page 7 cited a letter from M.S. Fish dated 7 January 1958. ref Trout's Notes
- In seeds by TLC. Yamasato et al. 1972. Ref Trout's Notes
Phalaris spp.
- Bufotenine present in all fresh samples they examined but not in all dried samples and if so was present in considerably reduced amounts. Culvenor et at. 1964
- Bufotenine traces both in commercial material and in AQ-1 (Italy). HPLC by Fabio Calligris. Festi & Samorini 1994b ref trout's Notes
- Australian commercial - Bufotenine Trace. Baxter & Slaytor 1972b ref Trout's Notes
- (France) Bufotenine traces reported (HPLC) Festi & Samorini 1994b ref Trout's Notes
- (Portugal) Bufotenine Traces reported (HPLC) Festi & Samorini 1994b ref Trout's Notes
- (Portugal) Bufotenine Traces reported (HPLC). Festi & Samorini 1994b ref Trout's Notes
- (Romania) Bufotenine Traces reported (HPLC) Festi & Samorini 1994b ref Trout's Notes
- (France) Bufotenine Traces reported (HPLC) Festi & Samorini 1994b ref Trout's Notes
Phalaris tuberosa ( see P aquatica)
Phragmites spp.
- Bufotenine in Rhizome. Not quantified. Wassel et al. 1985 ref Trout's Notes
Umbellularia spp.
- Bufotenine presence - Shulgin & Shulgin 1997
Urtica spp.
- Bufotenine presence. Shulgin & Shulgin 1997
Virola spp.
- The listing of bufotenine for this species IS in error. We suspect that it stems from Holmstedt 's analys is of Epena claimed by Seitz to have originated from Virola. This is the lone claim of bufotenine in the genus and it is considered by most not to represent a Virola based snuff for this reason. We encountered one string of claims of bufotenine showing up as a minor base in analysis of this plant (Kawanishi et al. 1985 made the claim for this species citing Schultes & Holmstedt 1971 . Schultes & Holmstedt mention this in passing as part of an included quote taken from Corothie & Nakano 1969. Schultes & Holmstedt deliberately deleted the portion of the quote that implied that Holmstedt found this alkaloid in the species. Holmstedt analyzed epena snuff. Corothie & Nakano 1969's wording could be taken to mean that Holmstedt reported this but do not include a
reference (# 14) for the statement. The only paper of Holmstedt (#6 in their list) that they list is Holmstedt's analysis of Seitz's Epena snuff. [i.e. Holmstedt 1965). At no point during that particular study did Holmstedt analyze plant material of Virola sebifcra and detect this compound. Ref Trout's Notes
Humans and other animals
Coral
Paramuricea chamaeleon (Coral)
- 10 mg. of bufotenine isolated from 200 grams of coral Cimino & DeStefano 1978 ref Trout's Notes
Toads and frogs
(ref Trout's Notes)
- Names followed by plus sign(s) from Daly & Witkop 1971:
- + 1- 100 ug / gm of skin
- ++ 100- 1000 ug / gm. of skin
- +++ 1-10 mg / gm. of skin
Wieland et al 1934 and Wieland& Wieland 1937 Ch'an Su (Chinese phanmceutical preparation of toad venom) Isolated by Jensen & Chen 1932
Bufo sp. Wieland & wieland 1937
Bufo alvarius Wieland & Wieland 1937
- Cutaneous glands were found to contain 0.8 to 5 mg/gm.
- Non-glandular skin was found to contain 0.33-2.15 mg/gm Erspamer et al. 1965
- Up to 3 mg per gram of dried skin.
- Concentrations ranged from less than 0.1 mg to 5.0 mg per gram of large cutaneous glands (averaging 1.2 milligrams/gm. glandular tissue) and from 0.17-2.2 mg per gram (averaging 0.9 mg/ gram) in the rest of the dry skin. Erspamer et al 1967
- + Identified by Erspamer 1954 (Also listed in Erspamer 1961)
- +++ Isolated by Jensen & Chen 1932. Wieland et al. 1934 recovered 5.1 mg/g dried skin. (Also listed inErspamer 1961)
- +++
- ++ Isolated by Handovsky 1920. found to contain 510 ug/animals (0.3% in dry secretions. Females: 90 ug/animal (0.33% in dry secretions) by Wieland & Behringer 1941 . (Also listed in Erspamer 196 1)
- ++
- ++ Identified by Erspamer 1954
- Deulofeu & Rúveda 1971
- + Identified: Alvares Pereira & de Oliveira 1961. Isolated: Deulofeu & Duprat, 1944
- +
- ++
- Isolated by Ohno et al. 1961
- + Identified by Erspamer 1954
Also listed in Erspamer 1961
- +
- ++
- +
- +
- ++
- + (Despite the unsupported claims that this species is a good source of bufotenine, it possesses only low concentrations WHEN it is even present. For example: Cei et al. 1968 found it was present in only some collections and even when present it was sometimes only in some individuals.)
- ++
- +
- +
- +++ Isolated by Deulofeu & Mendive 1938 & by Deulofeu & Duprat 1944
- +++
- +
- ++
- ++
- +
- ++
- +++ Identified by Erspamer 1959, who found 630 ug/gm of dried skin (0.06% in fresh skin.). (Also in Erspamer 1961: 640 ug/ gm of fresh skin.)
- Isolated: Jensen & Chen 1932 & 1936
- (also see Bufo bufo bufo) Handovsky 1920 (Also even earlier by Phisalix & Bertrand 1893 but they did
not fully characterize.) lsolated by Jensen & Chen 1936.
- (0 & 100 ug/ gm: Roseghini et al 1976)
- (0 & 20 ug/ grm: Roseghini et al 1976)
- ++ (0.75 mg/ grn: Roseghini et al. 1976)
- (0.87 & 2.3 mg/ gm: Roseghini et al. 1976)
- + (25 ug/ gm: Roseghini et al. 1976)
- + (5 ug/ gm: Roseghini et al. 1976)
- + (0 & 0 & 8 ug / gm: Roseghini el al. 1976)
- +++ (0.75-7 mg / gm: Roseghini et al 1976)
- + (10 & 15 ug/gm: Roseghini et al. 1976)
- + (10 & 60 ug/ gm: Roseghini et al 1976)
- (35 ug / grm: Roseghini et al. 1976)
- +++
- +
- (85 ug / gm: Roseghini et at. 1976)
- (10-25 ug/ gm: Roseghin.i et al. 1986)
- +
Humans
(ref Trout's Notes)
- Bufotenine is found in normal human blood and urine; Franzen & Gross 1965
- Angrist et a/. 1976 found that while the overall values were higher in their patient group (especially patients with acute psychosis, in women patients and in those patients with a high suspiciousness rating), there was no statistically significant difference between bufotenine blood levels of psychotic patients and normal subjects.
- Karkkainen & Raisanen 1992 also detected endogenous bufotenine in normal humans. See also Raisanen & Karkkainen 1979.
- Narasimhachari et al. 1971b reported it was more common in psychotics than normals; also see Sitaram's papers.
- For more references on its reported occurrences in humans: See Davis 1989.
Extraction teks
Dry tek
IPA extract
Yopo seed extraction
Dosages and consumption methods
History of usage
Analysis of bufotenine
Scientific publications
Other links of interest