Category:DMT
Contents
- 1 Brief overview - What is DMT?
- 2 Chemical and physical properties
- 3 Effects
- 4 Pharmacology, toxicity and general safety
- 5 Plants containing DMT
- 5.1 Acacia spp.
- 5.2 Anadenanthera spp.
- 5.3 Anthirea spp.
- 5.4 Arundo spp.
- 5.5 Delosperma spp.
- 5.6 Desmanthus spp.
- 5.7 Desmodium spp.
- 5.8 Diplopterys spp.
- 5.9 Erigonum sp.
- 5.10 Evodia spp.
- 5.11 Justicia spp.
- 5.12 Lespedeza spp.
- 5.13 Limona spp.
- 5.14 Mimosa spp.
- 5.15 Mucuna spp.
- 5.16 Osteophloem spp.
- 5.17 Pandanus spp.
- 5.18 Petalostylis spp.
- 5.19 Phalaris spp.
- 5.20 Phragmites spp.
- 5.21 Psychotria spp.
- 5.22 Testulea spp.
- 5.23 Vepris spp.
- 5.24 Virola spp.
- 5.25 Zanthoxylum spp.
- 5.26 Humans and other animals
- 6 Extraction Teks
- 7 Dosages and consumption methods
- 8 History of usage
- 9 Analysis of DMT
- 10 Scientific publications
- 11 Links of interest
Brief overview - What is DMT?
NN-Dimethyltryptamine or DMT for short is an short acting psychedelic entheogen which allows a persons consciousness to voyage into the most incredible dimensions, visions, thoughts and experiences imaginable.
It is one of the most powerful yet mysterious psychedelics in existence, but in the opinion of many users, to classify DMT as merely a drug would be doing it a great injustice as DMT seems to some as a trans dimensional key into places and vistas so profound and awe inspiring that it raises many new questions regarding the nature of reality and our place within it. Nevertheless, it is important to realize that the experience may be very difficult for some to integrate, and a great care and respect is necessary to use it. Please check Health and Safety section for more info.
DMT exists naturally in every human being and also throughout the plant and animal kingdoms. It occurs naturally in many mammals, marine animals, trees, grasses, flowers and shoots.
DMT is closely related to serotonin, the naturally occurring neurotransmitter that psychedelics affect so widely. The pharmacology of DMT is similar to that of other well-known psychedelics. It affects receptor sites for serotonin in much the same way that LSD, psilocybin, and mescaline do. These serotonin receptors are widespread throughout the body and can be found in blood vessels, muscle, glands, and skin.
There are a number of ways to acquire this entheogen. The first and most difficult way is to have some substantial chemistry knowledge and experience and actually synthesize pure DMT in a laboratory. This a rather tricky and time consuming process and requires access to some rather obscure and hard to acquire chemicals.
The most common and easiest method to acquire DMT is to extract it from the various plant species that contain the compound. The various plants and extraction techniques can be found further down this page.
Chemical and physical properties
For solubility, instabilities, etc, check the DMT Chemical and Physical Properties WIKI
Effects
Subjective effects
Depending on the dosage and form of ingestion, the effects of DMT can range from a multitude of sensations, from light, subtle perceptual changes, to bizarre, beautiful and even 'impossible' visions , and to literally jaw hanging awe as one is propelled into other dimensions of existence where human language and logic cannot even begin to describe or comprehend.
There have been a few attempts to define different levels and types of experience. Psychedelic Monographs and Essays Psychedelic Monographs and Essays discusses different levels of a DMT experience.
The Hyperspace lexicon project is an attempt to create a new vocabulary to try to describe the DMT realm.
Another way to get a small glimpse of the effects it can generate from a subjective point of view, read the areas of the forum dedicated to experience reports (1, 2)
Physical effects
Increase in heart rate and blood pressure. Severe mydriasis. Very even breathing. Trance like effects.
Combinations / Synergies / Interactions
Below are some of the effects of interaction between DMT and other substances:
- With harmine/Harmaline: Harmalas activate DMT orally by temporarily inhibiting MAO-A. Harmalas (sublingual, oral or vaporized) also extend the duration of a vaporized DMT experience, and can help some remember better the experiences with the slower comedown, but specially in higher doses harmalas might make the experience heavy/foggy and not allow for a clean breakthrough. Some people enjoy it, others prefer vaporizing without harmalas. The ones that do like it, usually prefer in smaller/medium doses of harmalas instead of higher doses.
- DMT & Harmala: Dosage and Routes of Administration (smoking, oral, sublingual, snorting...)
- With cannabis: DMT vapor is cool. Cannabis smoke/vapor is hot. This can make for difficult hits as well as difficult to hold hits when using DMT in a cannabis changa or with some cannabis herb. When used before launch cannabis can help or hinder the process of dealing with anxiety. Also, when used before launch, many report damped down visuals. Finally, with this combo, memory of the trip can be even more difficult. Cannabis is very pleasant during the comedown from DMT.
- With mushrooms: DMT vaporized during the mushroom experiences are very intense, and the preferred combination of some. Trout reports some to find it disorienting.
(Pandora) Very intense has included heavy audio hallucination along with open eyed full on breakthrough visuals of an "attack" nature as well as cognitive delusion for me.
- With LSD: Many people really like the experience and find it can be really beautiful and ecstatic. Others find in spite of beautiful visuals, the LSD acts as an anchor and prevents breakthrough.
(Pandora) - One of the most intense and profound combinations. Takes me a higher dose than usual of DMT to breakthrough - benzyme says due to competition at receptor site. The visuals are an order of magnitude or three greater than DMT alone and open eyed can turn the world into a vibrating, synergistic, Legoland. During the comeup it eases me through the transitions and plunges me right into heavy LSD tripping (when DMT fades). During peak it is almost guaranteed ego death/mystical. During comedown, ratchets my LSD trip back up (when DMT fades) and frankly is very erotic.
- With mescaline: As with LSD
(Pandora) - Mescaline and DMT yield more organic and less Lego-like visuals for me with generally darker colors than the LSDMT combo. I find that the Mescaline DMT combo radically extends the peak DMT effects for me.
- With Chlorpromazine: Diminishes effects (Trout's Notes)
- Benzodiazepines: The psychedelic community generally considers that aborting a bad trip with such substances is counterproductive and can generate long lasting psychological issues. Gentle comforting approach is generally recommended and thought to be of great effectiveness. Being reassuring and calm, maybe a warm blanket or a tea or a juice, can go much farther and deeper in resolving a psychedelic crisis situation, as it has been shown in several real-world observations. In any case Trout reports 0.5-1mg of Alprazolam can smooth an agitatedly rough or unpleasant trip or perception of body load, or enabling of sleep at the end of an intense session involving stimulant psychedelics or "party" drug combinations. (Trout's Notes)
- With MDMA: Mixed responses. Check this thread for some more info. Do NOT mix changa containing MAOIs with MDMA, and certainly neither oral MAOIs and MDMA.
- With LSD+MDMA (candy flip): Launching is much more comfortable than with LSD alone, though effects are mixed as with DMT and MDMA alone and Pandora has purged on this combo twice.
- With 2CB: Intensely insightful with lasting impact. See yourself and your memories in a new light.
- With 5-meo-MIPT: Pandora found this similar to the LSD combo without the ultimate ego-death/mystical peak.
- With Ketamine: Friendly, thickly visual and seductive combination. Ketamine makes for a completely relaxed, warm, anxiety-free launch. Pandora prefers a non-dissociative dose of K (under 50 mgs) and a number of changa hits over one hour for the ideal DMT/K session. Memories are elusive here.
- With MXE: Same as with Ketamine but more so. Pandora finds DMT to be best on the comedown but needs more assays. Warm, comfy, extended stays in hyperspace with things moving slowly or not at all. A sense of floating and moving in curves in a zero-G environment. Strong feeling of "instant integration" when the trance breaks.
Pharmacology, toxicity and general safety
Pharmacology
DMT is inactive when taken orally, unless if ingested together with MAOI.
After intramuscular injection it is rapidly metabolised primarily into indol-3-ylacetic acid. About 33% of the dose is excreted in urine in 6 hours as free and conjugated (glucoronide) indol-3-ylacetic acid. Less than 0.1% of the dose is excreted unchanged in the urine in 24 hours (Clarke's second, 1986)
DMT is an agonist of serotonin 5-HT2a, 5-HT2c and 5-HT1a
For specific information about DMT/Ayahuasca pharmacology, read these papers:
- Ayahuasca pharmacology part 1 Jordi Riba
- Ayahuasca pharmacology part 2 Jordi Riba
- Human pharmacology ayahuasca
- DMT psychopharmacology
Safety
For info on DMT safety, please reffer to Health and Safety section
Plants containing DMT
The following is a list of plants known to contain DMT. Plants no containing other tryptamines can be found in the page of each tryptamine, such as 5-MeO-DMT, Bufotenine, or other Alkaloids.
In some of the plants in the following list, the DMT content may be very small or it may be present together with other potentially unwanted alkaloids. Please research well before extracting from some plant, and be sure you have your desired alkaloids only when bioassaying from a new plant. For more information,
Acacia spp.
Acacia acuminata 0.6-1.8 DMT bark, up to 1.2% leaves https://www.dmt-nexus.me/forum/default.aspx?g=posts&m=265884#post265884 net reports])
Acacia albida DMT (Shulgin, Tikhal). No reference in Tikhal, but several underground extractions in Israel https:(Nen & chocobeastle)
Acacia alpina DMT-Like effects (two bioassays)
Acacia angustissima very low amounts: 1.2-2.8 mg/kg DMT (Mcksweeney et al 2005)
Acacia baileyana Trace amounts in seeds, Unconfirmed (tlc by J. Apleseed, ref. Trout's Notes). tryptamine and βcarbolines, in the leaf, Tetrahydroharman (TIHKAL)
Acacia blakei DMT reagent+ve, 1 report effects (Nexus)
Acacia binervata Positive for reagent (Nen, 2001)
Acacia colei 1%+ in bark (ABC radio, different net reports)
Acacia confusa 1.15% DMT in rootbark (Liu et al 1977 ref. Trout's Notes), 0.01% DMT in dry stem-bark (Arthur et al 1967)
Acacia cornigera Presence of DMT in bark indicated but details lacking, needs confirmation (Ratsch 1998 ref. Trout's Notes)
Acacia difformis Traces of DMT in leaf. Xanthydrol. Both pinnate leaves and phylodes tested separately. 2 year old plant. (Trout's notes)
Acacia floribunda 0.3-0.8% DMT, NMT, tryptamine, harman (S. Voogenbreinder; numerous net reports and bioassays)
Acacia laeta DMT in the leaf has been reported but in error due to misreading of Wahba % Elkheir 1975 who reported negative results (Trout's Notes)
Acacia longifolia 0.2% tryptamine in bark, leaves, some in flowers, phenylethylamine in flowers (Hegnauer 1994) DMT in plant (Lyceaum), but trout claims reports are in error due to methodology.{this statement needs clarification] Daniel Siebert found trace amounts of DMT in aerial parts in CA but did not publish information (ref. Trout's Notes) Voogelbreinder 2009 and Nen [dmt-nexus] found several confirmed findings of DMT at 0.2-0.3% in bark. White (1951) found 0.2% tryptamine in bark, but his other previous findings of tryptamine turned out to be DMT (e.g. acuminata, floribunda) * Var sophorae: 0.6%DMT,5meoDMT,Tryptamine,Bufotenine,Gramine ,Cinnamoylhistamine, n-dec-3enoylhistamine[entheogen review 1995], some strains very little alkaloids
Acacia maidenii Bark of A. maidenii contains 0.6% of N-methyltryptamine and DMT in the proportions approx. 2:3 (Fitzgerald & Sioumis 1965) Narrow-leaf variety high alkaloid content.
Acacia melanoxylon DMT in the bark and leaf, less than 0.02% total alkaloids (Hegnauer 1994)
Acacia mellifera DMT reported but probably error (Trout's Notes)
Acacia mucronata 0.4% DMT,NMT,Trptamine, betacarbolines (Snu Voogenbreider Garden of Eden citing 'E', dmt-nexus.me)
Acacia neurophylla DMT (bark), harman, norharman (leaves) [S. Voogenbreinder Garden Of Eden citing 'Jeremy']
Acacia nubica 0.0016% in dry leaf (Wahba Khalil & Elkheir 1975 ref Trout's Notes)
Acacia obtusifolia 0.4 to 0.5 % DMT/NMT in the dried bark (Csiro 1990) 0.15-0.6% DMT,NMT(1:2)plus trace betacarboline in bark, 0.06-0.2% leaves (Southern Cross University comissioned test 2001) 5-MeoDMT & bufotenine in some loctations (E., Entheogen Review 1995-6; Trout's Notes 2005-10) Is not fast growing in the wild and is under threat of serious overharvesting. Is NOT considered a weed as previously stated here, and will become rarer if wild seed populations exploited further.(Nen, original bioassay subject)
Acacia oerfota Less than 0.1% DMT in leaf (Ott)
Acacia oxycedrus 0.4-0.5% alkaloid stem-bark DMT-like effects [dmt-nexus.me]
Acacia phlebophylla Rare, limited to one area 0.3% DMT in leaf, NMT (Rovelli & Vaughan 1967)
Acacia podalyriaefolia Tryptamine, NMT in the leaf (Trout's Notes) 0.5% to 1.8% DMT in fresh bark, phenethylamine trace amounts (Hegnauer 1994). This claim has not been replicated, all low yielding reports around the net.
Acacia polyacantha 0.004% DMT in leaf (Wahba Khalil & Elkheir 1975 ref Trout's Notes)
Acacia pycnantha Small amount DMT leaf (Voogelbreinder 2009; Hegnauer 1994); rumored 0.5% tryptamines bark (dmt-nexus).
Acacia retinodes 0.2% alkaloid (unknown) (Roveli 1967); 0.5%DMT, NMT, nicotine?,(Pflanzentabelle APB (German) Less than 0.02% total alkaloids found (Hegnauer 1994)
Acacia rigidula DMT, NMT, tryptamine, amphetamines, mescaline, nicotine and others, but this report is in serious question due to reference standards problems (Clement et al 1998)
Acacia senegal 0.003% DMT, in the leaf (Wahba Khalil & Elkheir 1975 ref Trout's Notes)
Acacia simplicifolia 0.81% DMT in bark, 0.007% in twigs, co-occurng with 1.44% NMT in bark and 0.29% in twigs (Poupat et al 1976)
Acacia tortilis Erroneously reported (Trout's Notes)
Acacia sieberiana Erroneously reported (Trout's Notes)
Acacia victorae Aerial parts of 1 year old seed grown material (unconfirmed), Good banding (J Appleseed 1995 ref Trout's notes)
Anadenanthera spp.
- Conflicting reports. Most accounts only found bufotenine in seeds, but some reports claim presence of 5-MeO-DMT and/or DMT (Trout's Notes)
- (Argentina) - Snuff believed to be derived from A. colubrina was found with all 3 aforementioned alkaloids but its not clear (Torres et al 1991 ref Trout's Notes)
- (Argentina) - DMT was main or sole alkaloid in pods (Iacobucci & Ruveda 1964)
- (Argentina) - Only bufotenine in seeds (Trout's Notes)
- (Florida and Brazil) Not detected in seeds, detected in pods as sole alkaloid. Florida material weaker. 1.5-2% total alkaloids in seeds, pods weaker but only DMT found (Fish et al 1956)
- var Cebil (Argentina) 0.06% in seeds from Misión Wichi and 0.05% in pods from Salta but not detected in seeds from Salta. Traces detected in bark from Cerro San Bernardo (Torres & Repke 1996 ref Trout's Notes)
- DMT in seedpods (sole alkaloid present) (Iacobucci & Ruveda 1964)
- Seed pods contain dimethyltryptamine and the seeds bufotenin, bufotenin oxide, and oxide of dimethyltryptamine (GRANIER-DOYEUX 1965)
- (Boa Vista, Brazil,N24625)DMT at 0.0004% dry bark (1% of total alkaloids), 0.0059% dry leaves (49% of total alkaloids), plus 5-MeO-DMT at 0.025% dry bark and 0.006% dry leaves (Agurell et al 1969)
- (Puerto Rico and Brazil 1955) DMT was sole alkaloid in pods. Not detected in seeds: Puerto Rican material gave variable results. (Reported a total alkaloid concentration of 1.6% in the seeds. No indication was given of the actual amount of pure alkaloids. The pods were weaker but contained only DMT.)(Fish et al 1956)
- (Puerto Rico, 1948) Seeds - DMT (with Bufotenine) (Holmstedt & Lindgren 1967 ref Trout's notes)
- (Rio Branco, Brasil, 1953) Seeds - DMT (with 5-MeO-DMT) - (Holmstedt & Lindgren 1967 ref Trout's Notes)
- (Colombia, 1956) Bark - DMT (With NMT, 5-MeO-NMT and 5-MeO-DMT) (Holmstedt & Lindgren 1967 ref Trout's Notes)
- (San Juan, Puerto Rico Mar. 1975, N26363) Seeds (no quantification, 19% of alkaloid in 1975 analysis, only bufotenine found in 1977 analysis of same material). Collected in the hill behind El Comandante horse-racing track. La Carolina Barrio St. Just, near San Juan, Puerto Rico (Schultes et al 1977 ref Trout's Notes)
- (San Juan, Puerto Rico Dec. Dec. 1972, N26363) Immature seeds: 0.16% DMT (75% of 209 mg of total alkaloid/100 gm dry). Seedlings 0.001% DMT 4% of 25 mg of total alkaloid/ 100 gm dry. Pods without seeds 0.001% DMT (8% of 13 mg of total alkaloid/ 100 gm dry). Leaves 0.013% DMT (12% of 107 mg of total alkaloid/ 100 gm dry). Twigs 0.0019% DMT (5% of 38 mg of total alkaloid/ 100 grm dry). Bark (0.41% total alkaloid) 0.02% DMT (5% of 410 mg of total alkaloid/ 100 gm dry.) Roots (0.69% total alkaloid) 0.014% DMT (2% of 699 mg of total alkaloid/ 100 gm dry) (Schultes et al. 1977 ref Trout's Notes)
- (Boa Vista, Brazil, N24625) Leaves 0.00637% DMT (49% of 13 mg of total alk./ 100 gm dry) Bark 0.00042% DMT (1% of 42 mg of total alk. /100 gm dry) (Schultes et al 1977)
- (Abbott Lab, San Juan, Puerto Rico 1948) Seeds- 0.009% [9 mg of DMT/ 100 gm; Sole alkaloid) (Schultes et al. 1977 ref Trout's Notes)
- (J. Yde, 1964, H4685) Seedlings- 0.001% (1 mg ofDMT/ 100 gm; Sole alkaloid. (Schultes et al 1977 ref Trout's Notes)
Anthirea spp.
- DMT in roots with gramine, 6-Methoxy-2-methyl-tetrahydro-B-carboline, and N,N-Methyl-3-indolyl-methyl-5-methoxytryptamine. (Weniger et al. 1995 ref Trout's Notes)
Arundo spp.
- 20mg from 200grams of dried plant (compared to 520mg gramine per 200g plant!) (Ghosal et al 1971b)
40 mg per 700g rhizome (Dutta & Ghosal 1967 ref Trout's Notes)
- Plants analyzed in india were found with alkaloids. Plants from USA were not found with DMT based alkaloids (Trout notes)
- Numerous essays did not reveal DMT, but other indolic alkaloids (Appleseed & Trout ref Trout's notes)
Delosperma spp.
(all Delosperma are TLC assays by Appleseed, ref Trout's notes)
- DMT present in undetermined amount. 5 positive assays over a 15 month period. (Xanthydrol-1 and Ehrlich 's-4) (Sept.,Nov, Dec.) ( 1993-5) Not observed in May assay.
- Sept., Nov. and Dec. assays. 4 positives for DMT over a 25 month period. (Xanthydrol-2 and Ehrlich's-2) (Sasha was unable to confirm this using GC-MS on material purchased from Home Depot in Spring.)
- DMT positive Nov. 1994, 1995 (2, one year apart) also Sept. 1996 (1-Ehrlich's and 2- Xanthydrol)
- DMT - Nov. 1995 assay. Faint (Xanthydrol)
- (Yemen) - Nov. 1995 assay. Faint (Xanthydrol)
- Nov. 1995 and Dec. 1994 assays. Weak DMT band. (Xanthydrol and Ehrlich's)
- Sept. 1996 assay. (Xanthydrol) Co-occurrence with 5-MeO.
- Dec. 1994 (Ehrl ichs) and August 1995 harvest. Good DMT band (co-occurring with 5-MeO-DMT) (Dec. 1994 harvest; same material retested with Xanthydrol in 1996) Co-occurrence also observed in August and December 1995 harvests assayed in Sept 1996 (Xanthydrol)
- Nov. 1995 assay faint (not present in May assay ) (Xanthydrol) Sept 1996 assay decent. Xanthydrol. No alkaloid observed in Sept 1996 D. pergamentaceum Rooilepel.
- Nov. and Dec. 1994 assay Faint (or was it 5-MeO-DMT'?) (Ehrlich's)
Desmanthus spp.
- 0.34% in Root bark (dried) and 0.01 % in Root wood (dried) (Thompson et al. 1987) Substantially less is usually encoun-
tered. Sometimes none. (Trout's Notes)
- 0.14% yield of alkaloid. Identified by Johnny Appleseed 1992. TLC also tested positive 1993- 1995.
- Isolated and Bioassayed as pharmahoasca by J. Appleseed on 28 Nov., 1992.
- Isolated from Central Texas material and bioassayed as partially crystalline free base. Identity confirmed in bioassays by others, 1994 (ref Trout's Notes
- some tested +/ more tested - TLC by J. Appleseed ,1992 (ref Trout's Notes)
Desmodium spp.
- Roots - Major alkaloid 0.087% by dry weight. Ed .: Procedure likely resulted in some loss. If all of their crude alkaloid and all of their picrate had been used they would have obtained 1.46 gm from 1.6 kg dry roots. i.e. - 50 gm of roots for a 45 mg equivalency. Co-occuring with Bufotenine N-Oxide as minor root alkaloid (0.03%; 496 mg from 1.6 kg) (Ueno et al. 1978)
- Stem - DMT was minor alkaloid 0.0035%; 380 mg from 10.75 gm of stems). Co-occurring with Bufotenine, the major alkaloid in stem (0.04% by dry weight; If they used all of their picrate they would have recovered 4.3 gm of bufotenine base from 10.75 kg of stems.), plus bufotenine n-oxide (0.004%; 447 mg from 10.75 kg of stems. (Ueno et al. 1978)
- Aerial parts [? gm. of thick oil..:.. 0.41 grm DMT (latter as chloroform soluble acetate) obtained from 1 kg of fresh wet material. Banerjee & Ghosal 1969)
- Green Plant (Stem and Leaf) Ghosal et al. 1972a and Ghosal & Bhattacharya 1972; Green material bas 3X more alkaloid than if dried.
- Roots 0.38 gm DMT. from 1.6 kg. of dried roots. i.e.0.02% DMT (Banerjee & Ghosal 1969)
- Seeds - amount not given (Ghosal & Bhattacharya 1972 ref Trout's Notes)
- Fruit - amount not given (Ghosal et al. 1972a)
- Leaves ( 0.004% in dry leaf: 82 mg from 2 kg.) Ghosal et al. 1972a
- Roots (Minor alkaloid) Ghosal et al. 1972a
- Whole plant (DMT as minor alkaloid) Ghosal & Mukherjee 1964
- Stem and leaf of young seedling - 0.074% DMT by dry weight; 62% of 0.12% Total alkaloid (Ghosal et al 1972c ref Trout's Notes)
- Stem and leaf of mature plant - 0.294% DMT by dry weight; 21% of 1.4% Total alkaloid (Ghosal et al. 1972c ref Trout's Notes)
- Root of young seedling - 0.27% dry weight; 73% of 0.37% Total alkaloid - (Ghosal et al. 1972c ref Trout's Notes)
- Root of mature plant - 0.451% by dry weight; 41 % of 1.1% Total alkaloid - [Also, in same paper: 1.8 kg dried roots yielded 0.7g + 0.09 gm; i.e. 0.043%. (Ghosal et al. 1972c)
- Fruit (green) of mature plant - 12% of 0.01% Total alkaloid; ~0.001% by dry weight - (Ghosal et al. 1972c ref Trout's Notes)
- Seeds (ripe) of mature plant - 4% of 0.02% Total alkaloid; 0.001% by dry weight - (Ghosal et al. 1972c ref Trout's Notes)
- Root, stem-leaf and fruit - Amounts not given - (Ghosal 1972a)
- DMT-N-oxide, roots (Ott)
Diplopterys spp.
Diplopterys cabrerana (sometimes mislabelled as Banisteriopsis rusbyana, even though they are NOT the same)
- Leaves - DMT, traces of bufotenine ([http://www.crfdl.org/Mckenna, 1984)
- Leaves - 467mg DMT per 100g dry leaves, co occuring with trace amounts of NMT, Bufotenine, 5-MeO-DMT and MTHBC (Agurell et al 1968)
- Leaves - DMT as only significant peak (Endlessness 2011)
- Leaves - Only alkaloid present at 1.46% (1.33%-1.75% spectrophotometer estimate) - (Der Marderosian et al 1968a, ref Trout's Notes)
- Leaves - 1.3% DMT. Alkaloid content "largely DMT' (eastern Ecuador) (Der Marderosian et al 1968b ref Trout's Notes)
- Leaves - DMT was the major base in the leaves. 0.64% total bases comprised of DMT; 6.4 gm total bases per kg. He recovered 18 mg of DMT from 2.8
grams of leaves. (3 leaves) - He reported Beta carbolines in the stems, the major of which he believed to be harmine and smaller amounts of harmaline or 6-Methoxy-N,N-dimethyltryptamine. His extraction route would have been inefficient for harmine.] His material collected in Peru by Claudine Friedberg. (Poisson 1965 ref Trout's Notes)
- Stems - 166mg DMT per 100g dry stems, co-occurring with 3mg 5-MeO-DMT and 3mg MTHBC per 100g (Agurel et al 1968)
Erigonum sp.
DMT appears erroneously in the literature. The reference that was cited, Schroeder 1986, reported N,N-Dimethyl-tyramine
Eriogonum spp. include Buckwheat and Umbrella plants. There are about 150 spp. occurring as wild flowers and cultivars in the west and southwestern US and Mexico. Some are annuals and some are perennials. The only assay I have seen was positive for DMT but in traces. TLC by Appleseed (ref Trout's Notes)
Evodia spp.
Evodia rutaecarpa Hooker f. ex Thomas
- 0.00026% by dry weight in unripe fruit - 7.8 mg from 3 kg. (Yu et al. 1997)
Justicia spp.
- In leaf (Shulgin & Shulgin 1997)
- var stenophylla DMT in leaf ([1]). but Mckenna et al 1984 was unable to confirm. TLC bands corresponding to DMT, NMT and another high Rf alkaloid (Appleseed ref Trout's notes)
Lespedeza spp.
- Positive TLC assays in seeds, seed pods, stem-bark and roots. (Appleseed ref Trout's Notes)
- Seeds/seed-pods showed same alkaloids as stem-bark but darker and with 3-7 additional bands. (Seeds & pods harvested summer 1994) August stem-bark showed light band. (Appleseed ref Trout's Notes)
- Successful bioassay of 30 gm of red fall leaves reported by Wyrm; pers. comm. (ref Trout's Notes)
Roots harvested in December showed a positive for DMT and lighter for two other bands. (Appleseed 94-95, ref Trout's Notes) Some of these results used Ehrlichs spray and there may be confusion with 5-MeO-DMT in seeds and seeds/seed-pods.
- var. japonica:
- DMT in plant. (Goto et al. 1958 ref Trout's Notes)
- Major alkaloid in leaf and one of the main alkaloids in the root bark. Root bark showed higher concentration than leaves. (Morimoto & Matsumoto 1966)
- In leaf. (Morimoto & Oshio 1965 ref Trout's Notes)
Limona spp.
Limonia acidissima L. (= Limonia cremdata = Hesperethusa crenulata) (wood-apple, elephant-apple)
- 0.0045% DMT in dry stems. Many other compounds present; includi ng N-Acetyl-N-methyltryptamine, 3-Formylindo1e & 2-MTHBC
- Other plant parts apparently not tested. (Abu Zarga 1986)
Mimosa spp.
Roots
- 0.57% DMT - (Pachter et al 1959)
- 0.1-0.7% DMT (Gaujac et al 2012a)
- 0.9% DMT (DEA microgram bulletin)
- 1% DMT yield is commonly reported around the net, sometimes reaching up to +2%, and rarely on the low 0.2% end.
- Co-occuring with small amounts (3% of alkaloid fraction) of NMT and 2-MTHBC (Burnt's analysis of jungle spice)
- Co-occuring with small amounts of NMT, 2-MTHBC, 1-MTHBC, possibly 1,2-MTHBC, and traces of hordenine, N-Formyl-NMT, N-Methyl-Phenetylamine and Dimethyl-Phenetylamine (endlessness at jungle spice / mimosa hostilis analysis thread, 2011)
Stem
- (from mexico) - 0.03% DMT and 0.001% Serotonin (Meckes-Lozoya et al. 1990)
- (from brazil) - 0.1-0.9% DMT (Gaujac et al 2012a)
- 0.03% DMT (Meckes-Lozoya eta/. 1990)
Mixture of inner stem and root
- 0.7% crude extract, 0.3% pure (>95% pure DMT) by doing A/B and pulling on 60g mimosa with 5x50ml hexane, evap and one recrystallization. (Gaujac et al 2012b)
Leaf
- DMT, NMT, 2MTHBC, tryptophan - Not quantified. (Gardner et al 2012; Gardner pers comm)
Bark
- A/B extract - DMT, NMT, 2MTHBC, Hordenine, unknown potentially 1,2-DMTHBC. - Not quantified (Gardner et al 2012; Gardner pers comm)
- SPE extract - Same as above plus yuremamine and possible other yuremamine-like compounds. - Not quantified
- 1.6% DMT in rootbark, co-occuring with NMT (0.0012%), and hordenine (0.0065%) (Batista et al 1999)
- DMT in bark (Ott)
- This is included by a number of authorities. I can locate no published analysis on any material under this name. The references encountered (when a reference is even included) do not support the claim with analytical work. Usually the reference is Gonçalves de Lima who simply mentions that this plant is used for vinho da jurema. (ref Trout's Notes)
- Mimosa verrocosa is said by Da Mota 1991 to be used in making jurema, but to have sedative and not hallucinogenic effects. (Trout's Notes)
- Silveira Barbosa 1998 found it in use as a probable DMT containing brew in Brazil but (unlike M. hostilis] it appeared to be orally active as a hallucinogen only when an MAOI was coadministered. Her report of full activity with MAOI supports DMT's presence. (Trout's Notes)
Mucuna spp.
- DMT in leaf, stem, seed and root. Bhattacharya et al. 1971 (ref Trout's Notes)
- DMT in root, stem-leaf, and pod. Ghosal 1972 (ref Trout's Notes)
- 0.01% DMT in fresh leaves. Ghosal et al. 1971d (ref Trout's Notes)
- var. bennetti: Positive assay in seeds. Appleseed 1995(ref Trout's Notes)
- Leaves, seeds, stems and roots contain L-Dopa, Serotonin, 5-HTP, and Nicotine, as well as N,N-DMT, Bufotenine, and 5-MeO-DMT (Erowid)
Osteophloem spp.
- Small amount of DMT in bark of Schultes and Rodriguez No. 26126; Origin: Manaus, Brazil. One of 3 alkaloids in 0.62 mg of total alka-
loid from 100 grams of dry bark (Holmstedt et al. 1980 Ref Trout's Notes)
- Plowman, Schultes and Tovar # 7095; Origin: Pebas, Peru (Alpha-Helix 1977) assayed negative with Dragendorff and Ehrlich reagents. (Ref Trout's Notes)
Pandanus spp.
Hyndman 1984 cited personal communication from a D. Culvenor reporting DMT as a minor component among other alkaloids.
- DMT in nuts / seeds. Co-occurrence with harmine. TLC by J. Appleseed 1994. Not confirmed in 1995 assay. Harmine was still present but DMT was not detected in 1995. (ref Trout's Notes)
- DMT in nuts. Observed in hard core but not in fibrous outer nut. Harmine and another B-carboline (blue under UV) were present in both. TLC by Appleseed 1995 (ref Trout's Notes)
Petalostylis spp.
- var casseoides - Traces of DMT(Johns et al 1966 ref Trout's Notes)
Phalaris spp.
Phalaris aquatica syn. Phalaris tuberosa
- DMT is present in some clones and varieties. DMT in leaf (Baxter & Slaytor 1972; Culvenor et al 1964; Frahn & Illman 1973; Moore et al 1967; Mulvena & Slaytor 1982; Oram & Williams 1967 ref Trout's Notes)
- Clone R16 "Large" amount of DMT co-occuring with "trace" amount of 2MTHBC (Trout's Notes)
- Clone R36 "Trace" amount of DMT co-ocurring with "large" amounts of 2MTHBC (Trout's Notes)
- AQ1 - Highest DMT content in any Phalaris, 1% from grass grown in Italy (Festi & Samorini 1994 ref Trout's notes)
- Commercial var - Weak occurence reported by HPLC (Festi & Samorini 1994b ref Trout's notes)
- Australian Commercial - DMT 280nmol/100 seedlings ( 5-MeO-DMT 150nmol/100 seedlings) , 0.1% DMT dry weight of mature leaf (0.05% 5-MeO-DMT) co-occuring with traces of 5-MeO-T, 5-MeO-NMT (Mulvena & Slaytor 1983 ref Trout's Notes)
- GB 81 - Major base (Frahn & O'Keefe 1971 ref Trout's Notes)
- "High Alkaloid" - Major base (Frahn & O'Keefe 1971)
- JLF - Major base, 5-MeO-DMT & DMT in leaf sept 1995 TLC assay (J Appleseed 1995 ref Trout's Notes)
- Killer - DMT was predominant alkaloid in fall 1994, 5-MeO-DMT was predominant in summer/fall 1995 (J Appleseed 1995 ref Trout's Notes)
- Seedmaster - Major base (Frahn & O'Keefe 1971 ref Trout's Notes)
- Sirocco - 24 nmol of DMT per 100 seedlings (Mulvena & Slaytor 1983)
- DMT is present in some strains but NOT in most (Trout's Notes)
- (France) - Occurrence reported by HPLC (Festi & Samorini 1994b ref Trout's Notes)
- Ottawa Synthetic - Amounts not given, detected by TLC only in some of the samples (Wood & Clark 1971 ref Trout's Notes)
- (Portugal) - Extremely strong occurrence reported, sole alkaloid (Festi & Samorini 1994b ref Trout's Notes)
- (Algeria and greece clones) - Positive human bioassays (Dekorne 1997 ref Trout's Notes)
- USDA PI 202676 and 231044 - No detection, 5-MeO-DMT found instead (J Appleseed ref Trout's Notes)
- (Portugal) - Occurrence reported by HPLC (Festi & Samorini 1994b ref Trout's Notes)
- USDA PI 415833 - Occurrence reported by tlc (Appleseed ref Trout's Notes)
- USDA PI 284185 - Lower levels occurrence reported by tlc (Appleseed ref Trout's Notes)
- (Portugal) - Traces reported by TLC (Festi & Samorini 1994b ref Trout's Notes)
- (Romania) - Occurrence reported by TLC (Festi & Samorini 1994b ref Trout's Notes)
Phalaris stenoptera (= P. aquatica var. stenoptera)
- Variable amounts,Festi & Samorini 1994a cited Rendig et al 1970 as finding 0-60ug/ml of expressed juice (ref Trout's Notes)
- Syn Phalaris aquatica, read above
- Leaves and seedlings contain DMT, 5-MeO-DMT, and related compounds (Smith 1977)DMT - 0.100% (erowid)5-MeO-DMT - 0.022% (erowid)5-OH-DMT - 0.005% (erowid)
Phragmites spp.
Phragmites australis syn Phragmites communis
- DMT in rhizome. No details of amount included. (Wassel et al. 1985 ref Trout's Notes)
- TLC (as P. communis) by J. Appleseed showed it to be weak to absent. (ref Trout's notes)
Psychotria spp.
Psychotria alba Thought to contain DMT based on the fact that it is used interchangeably with P viridis by the UdV in Brazil. Published analysis is apparently lacking. Claimed to contain 60% as much as P. viridis. Independent analysis failed to detect DMT in at least one commercial strain. (Eel: pers. comm 2001, ref Trout's Notes)
- 0.65% DMT in dry leaf. [99% of 0.66% total alkaloid con-
tent by dry weight. "rami appant"; Culina Indians, Marcos. Collected 4 September 1968. Their specimens contained more alkaloid than the P. viridis they also analyzed. "practically all DMT". ([Rivier & Lindgren 1972])
- Many other assays have detected no DMT in this species, such as McKenna et al. 1984a, who analyzed DMCK #109yage-chacruna" from Tarapoto. and also Leal & Elizabetsky 1996 (ref Trout's Notes)
- 0.2% average DMT in dried leaves (Ott)
- May contain DMT due to the unsupported claim (by Duke & Vasquez Maninez 1993) of its application in Ayahuasca preparation but ana!ysis is apparently lacking. (ref Trout's Notes)
- Suggested to contain DMT due to the unsupported claim (by Duke & Vasquez Martinez 1993) of its application in Ayahuasca preparation but analysis is apparently lacking. (ref Trout's Notes)
- Bioassays indicate a strong presence of DMT. Personal communications with an unnamed source. (ref Trout's Notes)
Psychotria psychotriaefolia (Seem.) Standley
- Material erroneously identified. Actual identity was later
determined to be P. viridis. Was said to have DMT in leaf along with two non-indo1ic alkaloids. Der Marderosian et al 1969 (ref Trout's Notes)
- probable ID by R.E. Schultes; "falsa chacruna" (Shibipo) upper and middle Ucayali also by town dwellers in Iquitos.
0.8% total crude bases, with DMT was the major alkaloid. Percentage of DMT unspecified. (Urzua et al 1972 ref Trout's Notes)
Psychotria stenostachya Standi. May contain DMT based on unsupported claim (Duke & Vasquez Martinez 1993) of its application in preparing Ayahuasca. Apparently lacking analysis.
Psychotria viridis Ruiz & Pavon AKA "chacruna/chacrona" (Peru/Brazil), "sami ruca, "amurucapanga" (Ecuador))
- DMT in leaf (Der Marderosian el al. 1970 ref Trout's Notes)
- 0.34% DMT in dry leaf [99% of 0.34% total alkaloid content by dry weight. Traces of NMT and 2-MTHBC as minor alkaloids. DMT was absent from another specimen of this species. ([Rivier & Lindgren 1972])
- DMCK 21; Iquitos "chacruna - 0.16% DMT; 1.58 mg per gm dry weight (SD ± 0.3) in leaf, only alkaloid. (McKenna et al. 1984a ref Trout's Notes)
- DMCK 108; Tarapoto, "suija" - 0.10% DMT; 1.02 mg per gm dry weight (SD ± 0.04) in leaf, only alkaloid. (McKenna et al. 1984a ref Trout's Notes)
- DMCK 139; Pucallpa, "chacruna" - 0.12% DMT; 1.2 mg per gm dry weight (SD ± 0.17) in leaf. Traces of 2-MTHBC also present (McKenna et al. 1984a ref Trout's Notes)
- probable ID by R.E. Schultes; "chacruna" (Shibipo) upper and middle Ucayali also by town dwellers in Iquitos. - 0.24% total crude bases, DMT was the major alkaloid. Percentage of DMT unspecified. Said to be distinguishable from the 'false chacruna based on its profile of unidentified minor bases but the details were not included. (Urzua et al. 1972 ref Trout's Notes)
Testulea spp.
- Trace of in bark and root bark. DMT & 2 other alkaloids formed 10% of total. (Total alkaloid: 2.5% in Stem bark & 5% in Root bark) (Leboeuf et al 1977 ref Trout's Notes)
Vepris spp.
Vepris ampody H.Perr.
- 0.224% DMT in leaf. Co-occurring with Kokusagine, Dimethoxy-2,4-methy1-10-acridone, Evoxanthine and Phenacetamide. (Kan-Fan et al. 1970 ref Trout's Notes)
Virola spp.
- DMT found in Epena Snuff prepared by Tucano Indians: collected 1965, co-occurring with 5-MeO-NMT and 5-MeO-DMT (Holmstedt & Lindgren 1967 ref Trout's Notes)
- DMT found in Epena Snuff as prepared by Waica Indians (collected 1965), co-occuring with NMT and 5-MeO-DMT. (Holmstedt & Lindgren 1967 ref Trout's Notes)
- DMT found in Snuff prepared by Araraibo Indians: collected 1965. Co-occuring with 5-MeO-DMT. (Holmstedt & Lindgren 1967 ref Trout's Notes)
- DMT found in snuff obtained from Waica by George Seitz. DMT was a minor component. 5-MeO-DMT was the major. Bufotenine also observed as a minor alkaloid, casting doubts on the presumed botanical origin. (Holmstedt 1965 ref Trout's Notes)
- DMT found in Epena snuff No.24574; Origin: Rio Cauaburi, Brazil. 0.14% DMT i.e 1.43 mg per gm of snuff (20% of 715 mg. of total alkaloids / 100 gm. of snuff] (Agurell et al. 1969 ref Trout's Notes)
- DMT found in nyakwana snuff No. 24626; Origin: Tototobi, Brazil. 1.2% DMT i.e. 12.1 mg per gm ofsnuff (11% of 11,000 mg of total alkaloids/ 100 grm of snuff (Agurell et al. 1969 ref Trout's notes)
- DMT found in Paste: believed from a Virola sp. (No voucher; " oo '-koey"; La Chorrera. DMT 0.3 mg/ ml. 5-MeO-DMT was major. alkaloid at 1.19mg/ml. (McKenna et al. 1984a ref Trout's Notes)
- Bark collected in Manaus, Brazil during 1964. DMT co-occuring with NMT and 5-MeO-DMT (Holmstedt & Lindgren 1967 ref Trout's Notes)
- Leaves 0.149% DMT (Ott)
- No.24603; Origin: Manaus, Brazil was found with:
- Bark- 0.008% - 8 mg. of alkaloid/ 100 gm. of dry bark: Sole alkaloid DMT
- Roots- 0.0009% - 0.87 mg. of alkaloid / 100 gm. of dry roots: Sole alkaloid DMT
- Flowering shoots - 0.185% - 96% of 193 mg. of total alkaloids/ 100 gm. of dry flowering shoots
- Leaves- 0.15% - 149mg./ 100 gm. of dry leaves: Sole alkaloid DMT. (Agurell et al 1969 ref Trout's Notes)
Virola calophylloidea Markgraf
- DMT in bark and leaf. (Holmstedt et al. 1980 ref Trout's Notes)
- DMT in leaf. (Holmstedt et al. 1980 ref Trout's Notes)
- DMT in leaf. (Holmstedt et al. 1980 ref Trout's Notes)
- DMT in bark and leaf. (Holmstedt et al 1980 and McKenna et al 1984b ref Trout's Notes)
- DMT in bark. (Holmstedt et al. 1980 ref Trout's Notes)
- No.246 14; 0rigin: Manaus, Brazil was found with:
- Bark - 0.001% ( 1 mg./ 100 gm. of dry bark: Sole alkaloid DMT
- Root - 0.0004% (0.41 mg / 100 gm. of dry roots: Sole alkaloid DMT (Agurell et al 1969 ref Trout's Notes)
- DMT in leaf (McKenna et al 1984b ref Trout's Notes)
- DMT in bark (Holmstedt et al 1980 ref Trout's Notes)
- DMT in plant. Part and amount not given. (Lai et al 1973)
- DMT in bark, root and leaf (Agurell et al. 1969; Holmstedt et al. 1980 ref Trout's Notes)
- Alkaloids in bark and root, 95% of which is 5-MeO-DMT (Shulgin, TIHKAL)
- No.24612; Manaus, Brazil was found with:
- Bark- 0.19% (190 mg. / 100 gm. of dry bark: Sole alkaloid DMT
- Root- 0.001% (1.44 mg. / 100 gm. of dry roots: Sole alkaloid DMT
- Leaf- 0.09% (92 mg. / 100 grm. of dry leaves: Sole alkaloid DMT. (Agurell et al 1969 ref Trout's Notes)
- DMT in bark (Kawanishi el al. 1985, Corothie & Nakano 1969 ref Trout's Notes)
- DMK-40; Don Marcos no. 1 Paste: DMT 0.1 mg/ml, with NMT as the major alkaloid; present at 1.38 mg/ ml (McKenna et al 1984a ref Trout's Notes)
- DMT, 5-MEO-DMT in bark, roots, leaves and flowers (Ott)
- Bark- 0.0017% - 4 mg. of DMT in 235 gm. of bark. Co-occuring with NMT and 2 unidentified components. Leaves assayed negative. (Cassady et al 1971 & 1972. ref Trout's Notes)
- No.24595; Origin: Manaus, Brazil was found with:
- Bark- 0.13% - 52% of250 mg. total alkaloids/ 100 gm. of dry bark
- Root- 0.004% - 22% of 17 mg. of total alkaloids/ 100 gm. of dry roots
- Flowering shoots- 0.44% - 93% of 470 mg of total alkaloids/100 gm. of dry flowering shoots.
- Leaf- 0.04%- 99% of 44 mg. of total alkaloids/ 100 gm. of dry leaves. (Agurell et al 1969 ref Trout's Notes)
- No.24626; Origin: Tototobi, Brazil was found with:
- Bark- 0.003% in dry bark (5% of 65 mg. of total alkaloids/ 100 gm. of dry bark
- Leaf- 0.02% in dry leaves (98% of 21 mg. of total alkaloids/ 100 gm. of dry leaves (Agurel et al. 1969)
- DMT, 5-MEO-DMT in roots and leaves (Ott)
- No. 24613; Origin: Manaus, Brazil was found with:
- Traces of DMT in dry leaves. 1 mg per 100 gm
- Bark negative. 5-MeO-DMT in roots. (Agurell et al 1969 and Holmstedt et al 1980 ref Trout's Notes)
Zanthoxylum spp.
An odd side note: in TLC run (several times) on Zanthoxylum americanum bark, Appleseed saw a band that co-chromatographed with DMT but. turned a weird orange with Ehrlich's reagent.
- 0.09% leaf (dry weight) Many other compounds present. (Grina el al. 1982 ref Trout's Notes)
Zanthoxylum procerum Donn. Sm.
- DMT in leaf Ott cited Schroeder 1986. (Ref. Trout's Notes)
Humans and other animals
Coral, less than 5mg per 200g, Cimino & DeStefano 1978
Rats
Saavedra & Axelrod 1972 showed that MMT and DMT can be formed in rat brain and that an enzyme is present that is capable of performing this reaction. They also found that something else is present which inhibits this reaction.
Humans (ref Trout's Notes)
- Plasma concentrations of endogenous dimethyltryptamine are generally <0.001 mg/L. Fifteen male volunteers, between the ages of 26 and 48 years, were administered 2 mL/kg of hoasca tea, an Amazonian sacramental beverage. The tea alkaloid content was determined to be as follows: DMT 0.24 mg/mL, harmine 1.70 mg/mL, harmaline 0.20 mg/mL and tetrahydroharmine 1.07 mg/mL. The mean peak plasma concentration of dimethyltryptamine was 15.8 mg/L, reached after 107.5 min, which coincided with peak times of psychoactivity [Callaway et al. 1999].
- After IM injection of 0.7 mg/kg to 11 subjects, peak blood concentrations averaged 0.1 mg/L at 0.17 h, coinciding with the maximum psychoactive
effects [Kaplan et al. 1974].
- See the review of biogenic amines reported in human body fluids by Bruce Davis 1989.
- Clarke's Second Edition notes that natural endogenous concentrations in plasma are normally less than 0.001 ug/ml and that IM administration of 0.7 mg/kg resulted in an average concentration of 0.1 ug/ml at 0.17 hour. Said to be the time of maximum effect via this route. This is fascinating as it implies that strongly entheogenic activity is a result of elevation of the concentration by less than 100 times that of the naturally occurring baseline.
- DMT was found in normal human blood and urine by Franzen & Gross 1965.
- Sample analysis of human cerebrospinal fluid included DMT. Christian et al 1975.
- Found in cerebrospinal fluid of psychotics and normal people. Corbett et al 1978.
- Narasimhachari et al. 1971a found DMT in schizophrenics but not in normals.
- Narasimhacbari et al 1971b reported it in most psychotics but only 2 out of 20 normals.
- Smythies et al 1979 found it at wildly varying levels in both populations.
- Lipinski et al 1974 found it in some psychotics.
- Oon & Rodnight. 1977 thought they observed DMT in psychotics but did not positively prove. See also Oon et al. 1977 and his references.
- For additional references on the natural and potential occurrence of DMT in mammals and humans see:
Beaton & Morris 1984 Christian et at. 1976 & 1977 Raisanen & Karkkainen 1979 (in urine) Rosengarten & Friedhoff 1976 Saavedra & Axelrod 1972 Tanimukai et al 1970 Wyatt et al 1973 (found at variable levels in plasma of both psychotics & normals.) For references on occurrence in normal populations versus psychotics, see articles just mentioned , also those by Barker or Christian above, and Davis 1989 - Davis noted that those studies failing to find DMT were the ones that relied on less sensitive assay methods. Oon et al 1977 See discussion & references in Gillin et al 1976.
- Review: Rosengarten & Friedhoff I 976
Extraction Teks
For an overview on how extractions work, read the FAQ, and the Extraction Overview
A/B
STB
STB-A/B hybrid
Dry tek
Dosages and consumption methods
Smoked / Vaporized
Extracted DMT freebase can be vaporized for very potent effects that last around 10 minutes. DMT is ideally vaporized, as opposed to smoked. Vaporization is achieved by a controlled temperature that does not burn/combust DMT material (and potential impurities), but instead just makes DMT evaporate and be inhaled.
Vaporization is much smoother than smoking. Smoking leads to break down of DMT (and impurity) molecules into potential toxic nitrogen oxides (Trout's notes), so not only it is harsher but also there is a significant loss of actives.
Vaporizing can be achieved with improvised vaporizers such as The Inspirator mkII, or commercially sold vaporizing pipes such as the VaporGenie and oil/dab rigs [2].
Some methods, such as The Machine, if it's very carefully done, keeping the lighter farther away, one can also vaporize DMT, but due to lack of adequate buffer between the fire and the alkaloids, often will also generate combustion.
The GVG (Glass Vapor Genie) is usually touted as the most efficient/effective method of vaporizing DMT, however, recent exploration into dabbing DMT has proved to be at least as efficient and effective for many users. One advantage of dabbing DMT is that all of the vapor can be cleared in a single hit and is much smoother/less harsh than with the GVG. This is likely due to water cooling and increased distance of vapor travel. When dabbing DMT, however, one must ensure that the nail is sufficiently hot so that the Leidenfrost Effect can occur, resulting in the DMT being vaporized above the surface of the nail.[3]
Smoking is nonetheless still a popular way of ingesting DMT, and is often done by infusing herbs with the DMT, or smoking in a bong, with the DMT sandwhiched between thick layer of ashes or thin layer of herbs that serve to protect the DMT from fire (though there is still combustion, specially when using herbs).
Dosages are around 20-30mg for efficient vaporization methods, and with smoking methods can be around 50-60mg or even more....
Oral
DMT is only active orally when taking together with a MAOI. (FAQ for more info)
Dosages for DMT, considering MAOs are fully inhibited, vary wildly depending on person, probably due to metabolism in great part. They can go from 30 to 250mg! If its your first time, start on the lower end!
Another factor is whether one is ingesting a whole plant brew or purified extracts. Often in ayahuasca analysis the amount of DMT found is very small (20-30mg), but also often there is redosing in ayahuasca sessions, but also its possible other trace amount of beta-carbolines and alkaloids can improve MAO inhibition, or that other inactive plant substances can help protecting DMT from fast breakdown by any potential active MAO.
There are a few different ways to ingest it orally:
- Dissolved in acidic juice - Rolled inside a bit of smoking paper and swallowed like a pill - Put into 00 Capsules
Snorted
This is a method that gets very opposite responses from different people. For many, it hurts too much and isn't effective. For others it works well and pain/discomfort is tolerable, and the effects are worth it. It is unknown what possible health consequences snorting a basic alkaloid such as DMT can have on nasal passages, specially long term use, so we advice caution. There are some attempts to find less harsh ways, check threads below for more info:
Snorting Works! Preparations to make snorting more tolerable
History of usage
Analysis of DMT
To learn how analytical processes work, follow this link
Colorimetric reagents
References here
DMT
- α-Nitroso-β-naphthol-nitrous acid - Negative - (silica gel) - (23)
- - Weak brown (on paper) - (18 )
- Chloranil - No Reaction - (silica gel) - ( 8 )
- CNTF - Gray (light) - (silica gel) - ( 8 )
- Diazotized p-Nitroaniline - Very weak yellow - (on paper) - (18 )
- Dragendorff's - positive with spray - (silical gel) - (5)
- Red-Brown - (paper) - (18 ) - Orange - (silica gel) - (23)
- Ehrlich - Reddish purple - (as acetate on paper) - (26)
- Fluoranil - Purple - (silica gel) - ( 8 )
- Fluorescence with PENE - Violet under 254nm UV - (silica gel with PENE) - (24)
- HNS - No reaction - (silica gel) - ( 8 )
- HNO3 atmosphere - yellow - (silica gel) - (25)
- Iodine vapor - Red-Brown - (paper) - (18 )
- Iodoplatinate - Purple - (silica gel) - (25)
- Blue (silica gel) - (17)
- Iodoplatinate, acidified - Positive - (silica gel) - (5)
- Marquis - Yellow - (NA) - (13)
- Marquis - GreenYellow - (silica gel) - (7)
- Marquis - Orange->red - (NA) - (5)
- Mecke - Brown->red over time time. - (NA) - (13)
- Mandellin - yellow - (NA) - (13)
- Ninhydrin, acetic acid - No UV fluorescence - (acetate on paper) - (26)
- No visible color - (acetate on paper) - (26)
- NNCD - Weak orange - (on paper) - (19)
- p-DMAB, ethanol:sulphuric - Red solution, Violet when diluted with water - (5)
- p-DMAB-TS - Yellow - (pure compound) - (3, 27)
- p-DMAB, ethanolic - Purple - (pure compound) - (3)
- TACOT - Purple (light) - (silica gel) - ( 8 )
- TCBI - Brown-green - (silica gel) - ( 8 )
- TCNE - Brown (light and fading) - (silica gel) - ( 8 )
- TetNF - Brown (light) - (silica gel) - ( 8 )
- TNB - Yellow->Brown - (silica gel) - ( 8 )
- TNF - Brown (light) - (silica gel) - ( 8 )
- Van Urk - Blue - (silica treated with 0.1M KOH) - (16)
- Xanthydrol - Purple - (silica gel & celulose) - (15)
- Purple - (tlc & on paper) - (5, 20) - Pink - (on paper) - (21) - Lavender - (on paper) - (22)
LC / GC-MS
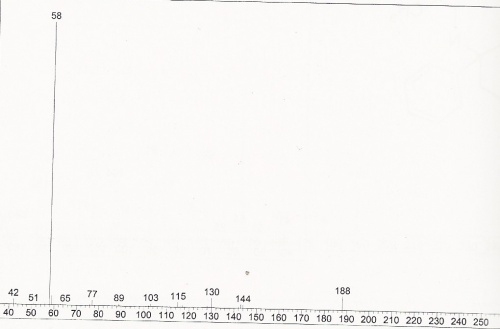
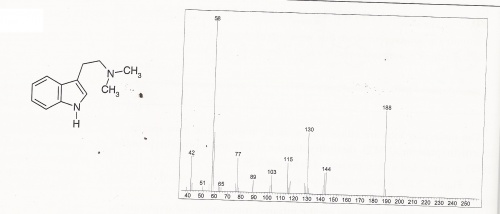
DMT Mass Spectra
DMT Mass Spectra (expanded)
Quantification
- Blood:
- GC Column: DB-1 fused silica capillary (30 m  0.32 mm i.d., 0.2 mm). Carrier gas: H2, 3 or 3.5 mL/min. Temperature: 230° and 280° . SID. Limit of
detection, 0.5 mg/L [Ishii et al. 1997].
- GC-MS Column: SE-30 (18 m  0.33 mm i.d.). Carrier gas: He, 2 mL/min. Temperature: 200° . EI ionisation at 70 eV, SIM acquisition mode. Limit of detection, 10 ng/L [Walker et al. 1979].
- Plasma GC Column: 5% phenyl methyl silicone capillary (12 m  0.2 mm i.d.,0.33 mm). Carrier gas: H2, 0.7 mL/min. Temperature programme: 70° for 1 min to 120° at 30° /min to 280° at 20° /min. Limit of quantification, 1.6 mg/L [Yritia et al. 2002].
- HPLC Column: Supelcosil LC-DB-8 (150 Â 4.6 mm i.d., 5 mm). Mobile phase: methanol : acetonitrile : 0.1 mol/L ammonium acetate (pH 6.9, 20 : 20 : 60), flow rate 2.0 mL/min. Fluorescence detection (lex1⁄4 232 nm, lem1⁄4 351 nm or lex1⁄4 340nm, lem1⁄4 495 nm). Limit of quantification, 2 mg/L [Callaway et al. 1996].
- Urine
- GC See Blood [Ishii et al. 1997].
- GC-MS Column: 1% OV-101 on 80/100 mesh Gas-Chrom Q. Temperature:190° . EI ionisation, MID. Limit of detection, 100 ng/L [Raisanen, Karkkainen 1979].
- LC-MS EI ionisation at 70 eV. SRM acquisition mode. Limit of detection, 2–10 mg/L [Bjornstad et al. 2009]. Column: Brownlee O Spheri-5 RP-18 (100 Â 1.0 mm i.d., 5 mm). Mobile phase: methanol : water (50 : 50) with 0.2% formic acid, flow rate 40 mL/min.
- ESI, MRM acquisition mode. Limit of detection, 0.1 mg/L [Forsstrom et al. 2001].
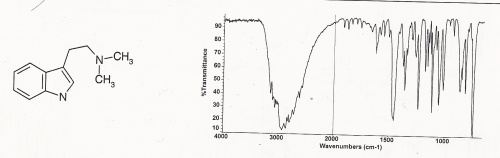
IR - Infrared
Other IR data (Clarke's second): Principal peaks at wavenumbers 743, 1113, 1235, 1050, 812, 1010 (KBr disk)
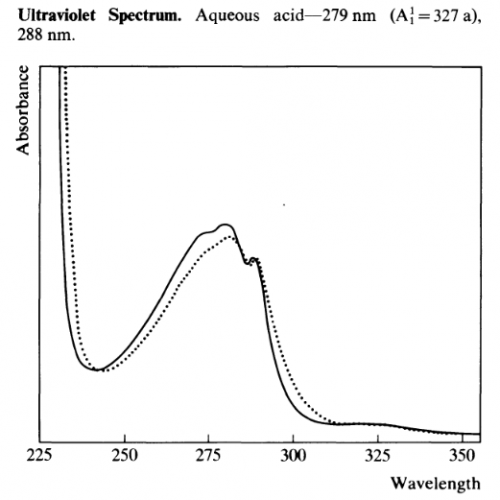
UV-Vis
Other UV-Vis data:
- λmax 222nm (log e 4.48), 277 (3.77) and 288 (3.75) Ghosal et al 1969
- λmax 222-224, 274 & 294nm Banergee & Ghosal 1969
- λmax 222, 277, 287 & 294 nm Ghosal & Banergee 1969
- λmax 274, 283, 291nm (refernce material) 275, 283, 291nm (isolated material) Fish et al 1955
- λmax (CH3OH): 220, 280, 290 (= 5500, 5600, 5000) De Moraes et al 1990
- λmax (EtOH): 226, 275 (sh), 279, 284, 293nm Grina et al 1982
- λmax 276, 282, 290nm
λmin 278, 287nm Martin & Alexander 1968
- λmax 275, 219 (0.1N NaOH)
- λmax 290, 276, 282 (EtOH) Sunshine 1981
- λmax of Xanthydrol reactive product (CHCl3): 510nm
λmin of Xanthydrol reactive product (CHCl3): 400nm Gander et al 1976
- Quantification. Average between: Ab@238/737*10000 = X ug/ml
Ab@308/296.1*10000 = X ug/ml
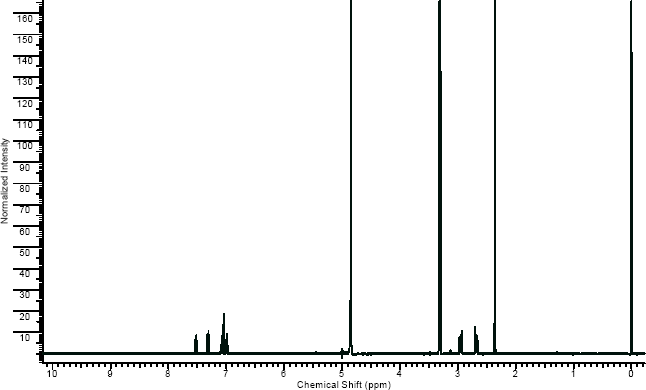
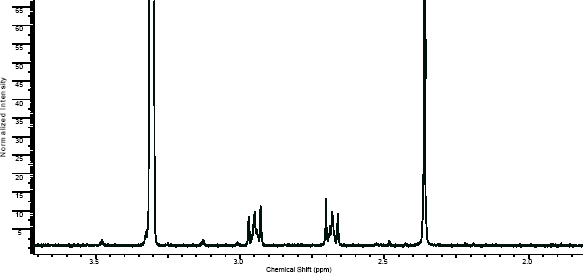
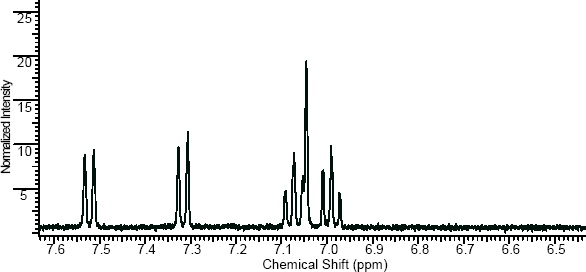
NMR
(NMR info source, method description and results discussion:Microgram bulletin volume 5, n14, pg6 )
Scientific publications
Links of interest
Pages in category "DMT"
The following 40 pages are in this category, out of 40 total.