Difference between revisions of "Category:DMT"
Endlessness (Talk | contribs) (→Diplopterys) |
Endlessness (Talk | contribs) (→Mimosa) |
||
| Line 338: | Line 338: | ||
[[Mimosa ophthalmocentra]] | [[Mimosa ophthalmocentra]] | ||
| − | * 1.6% DMT in rootbark | + | * 1.6% DMT in rootbark, co-occuring with NMT (0.0012%), and hordenine (0.0065%) ([https://www.dmt-nexus.me/forum/resource.ashx?a=8201 Batista et al 1999]) |
[[Mimosa scabrella]] | [[Mimosa scabrella]] | ||
Revision as of 13:38, 9 November 2011
Contents
Brief overview - What is DMT?
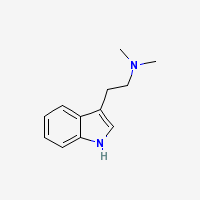
NN-Dimethyltryptamine or DMT for short is an short acting psychedelic entheogen which allows a persons consciousness to voyage into the most incredible dimensions, visions, thoughts and experiences imaginable.
It is one of the most powerful yet mysterious psychedelics in existence, but in the opinion of many users, to classify DMT as merely a drug would be doing it a great injustice as DMT seems to some as a trans dimensional key into places and vistas so profound and awe inspiring that it raises many new questions regarding the nature of reality and our place within it. Nevertheless, it is important to realize that the experience may be very difficult for some to integrate, and a great care and respect is necessary to use it. Please check [Health and Safety]] section for more info.
DMT exists naturally in every human being and also throughout the plant and animal kingdoms. It occurs naturally in many mammals, marine animals, trees, grasses, flowers and shoots.
DMT is closely related to serotonin, the naturally occurring neurotransmitter that psychedelics affect so widely. The pharmacology of DMT is similar to that of other well-known psychedelics. It affects receptor sites for serotonin in much the same way that LSD, psilocybin, and mescaline do. These serotonin receptors are widespread throughout the body and can be found in blood vessels, muscle, glands, and skin.
There are a number of ways to acquire this entheogen. The first and most difficult way is to have some substantial chemistry knowledge and experience and actually synthesize pure DMT in a laboratory. This a rather tricky and time consuming process and requires access to some rather obscure and hard to acquire chemicals.
The most common and easiest method to acquire DMT is to extract it from the various plant species that contain the compound. The various plants and extraction techniques can be found further down this page.
Chemical and physical properties
For solubility, instabilities, etc, check the DMT Chemical and Physical Properties WIKI
Effects
Subjective effects
Depending on the dosage and form of ingestion, the effects of DMT can range from a multitude of sensations, from light, subtle perceptual changes, to bizarre, beautiful and even 'impossible' visions , and to literally jaw hanging awe as one is propelled into other dimensions of existence where human language and logic cannot even begin to describe or comprehend.
There have been a few attempts to define different levels and types of experience. Psychedelic Monographs and Essays Psychedelic Monographs and Essays discusses different levels of a DMT experience.
The Hyperspace lexicon project is an attempt to create a new vocabulary to try to describe the DMT realm.
Another way to get a small glimpse of the effects it can generate from a subjective point of view, read the areas of the forum dedicated to experience reports (1, 2)
Physical effects
Combinations / Synergies / Interactions
Pharmacology, toxicity and general safety
Pharmacology
DMT is inactive when taken orally, unless if ingested together with MAOI.
After intramuscular injection it is rapidly metabolised primarily into indol-3-ylacetic acid. About 33% of the dose is excreted in urine in 6 hours as free and conjugated (glucoronide) indol-3-ylacetic acid. Less than 0.1% of the dose is excreted unchanged in the urine in 24 hours (Clarke's second, 1986)
DMT is an agonist of serotonin 5-HT2a, 5-HT2c and 5-HT1a
For specific information about DMT/Ayahuasca pharmacology, read these papers:
- Ayahuasca pharmacology part 1 Jordi Riba
- Ayahuasca pharmacology part 2 Jordi Riba
- Human pharmacology ayahuasca
- DMT psychopharmacology
Safety
For info on DMT safety, please reffer to Health and Safety section
Plants containing DMT
The following is a list of plants known to contain tryptamines. In some, the DMT content may be very small or it may be present together with other potentially unwanted alkaloids. Please research well before extracting from some plant, and be sure you have your desired alkaloids only when bioassaying from a new plant.
Acacia spp.
Acacia acuminata 1+% DMT (net reports)
Acacia alpina DMT-Like effects (two bioassays)
Acacia angustissima very low amounts: 1.2-2.8 mg/kg DMT (Mcksweeney et al 2005)
Acacia baileyana Trace amounts in seeds, Unconfirmed (tlc by J. Apleseed, ref. Trout's Notes). tryptamine and βcarbolines, in the leaf, Tetrahydroharman (TIHKAL)
Acacia blakey DMT-like effects (Nexus)
Acacia binervata Positive for reagent (Nen, 2001)
Acacia colei 1%+ in bark (ABC radio, different net reports)
Acacia confusa 1.15% DMT in rootbark (Liu et al 1977 ref. Trout's Notes), 0.01% DMT in dry stem-bark (Arthur et al 1967 ref. Trout's notes)
Acacia cornigera Presence of DMT in bark indicated but details lacking, needs confirmation (Ratsch 1998 ref. Trout's Notes)
Acacia difformis Traces of DMT in leaf. Xanthydrol. Both pinnate leaves and phylodes tested separately. 2 year old plant. (Trout's notes)
Acacia floribunda 0.3-0.8% DMT, NMT, tryptamine, harman (S. Voogenbreinder; numerous net reports and bioassays)
Acacia laeta DMT in the leaf has been reported but in error due to misreading of Wahba % Elkheir 1975 who reported negative results (Trout's Notes)
Acacia longifolia 0.2% tryptamine in bark, leaves, some in flowers, phenylethylamine in flowers (Hegnauer 1994) DMT in plant (Lyceaum), but trout claims reports are in error due to methodology. Daniel Siebert found trace amounts of DMT in aerial parts in CA but did not publish information (ref. Trout's Notes)
- Var sophorae: 0.6%DMT,5meoDMT,Tryptamine,Bufotenine,Gramine ,Cinnamoylhistamine, n-dec-3enoylhistamine[entheogen review 1995], some strains very little alkaloids
Acacia maidenii Bark of A. maidenii contains 0.6% of N-methyltryptamine and DMT in the proportions approx. 2:3 (Fitzgerald & Sioumis 1965)
Acacia melanoxylon DMT in the bark and leaf, less than 0.02% total alkaloids (Hegnauer 1994)
Acacia mellifera DMT reported but probably error (Trout's Notes)
Acacia mucronata 0.4% DMT,NMT,Trptamine, betacarbolines (Snu Voogenbreider Garden of Eden citing 'E', dmt-nexus.com)
Acacia neurophylla DMT (bark), harman, norharman (leaves) [S. Voogenbreinder Garden Of Eden citing 'Jeremy']
Acacia nubica 0.0016% in dry leaf (Wahba Khalil & Elkheir 1975 ref Trout's Notes)
Acacia obtusifolia 0.4 to 0.5 % DMT/NMT in the dried bark (Csiro 1990) 0.15-0.6% DMT,NMT(2:1)plus trace betacarboline in bark, 0.06-0.2% leaves (Southern Cross University comissioned test 2001) 5-MeoDMT & bufotenine in some loctations (E., Entheogen Review 1995-6; Trout's Notes 2005-10) Is not fast growing in the wild and is under threat of serious overharvesting. Is NOT considered a weed as previously stated here, and will become rarer if wild seed populations exploited further.(Nen, original bioassay subject)
Acacia oerfota Less than 0.1% DMT in leaf (Ott)
Acacia oxycedrus 0.4-0.5% alkaloid stem-bark DMT-like effects [dmt-nexus.me]
Acacia phlebophylla Rare, limited to one area
0.3% DMT in leaf, NMT (Rovelli & Vaughan 1967 ref Trout's Notes)
Acacia podalyriaefolia Tryptamine, NMT in the leaf (Trout's Notes)
0.5% to 1.8% DMT in fresh bark, phenethylamine trace amounts (Hegnauer 1994). This claim has not been replicated, all low yielding reports around the net.
Acacia polyacantha 0.004% DMT in leaf (Wahba Khalil & Elkheir 1975 ref Trout's Notes)
Acacia retinodes 0.2% alkaloid (unknown) (Roveli 1967); 0.5%DMT, NMT, nicotine?,(Pflanzentabelle APB (German) Less than 0.02% total alkaloids found (Hegnauer 1994)
Acacia rigidula DMT, NMT, tryptamine, amphetamines, mescaline, nicotine and others, but this report is in serious question due to reference standards problems (clement et al 1998 ref Trout's Notes)
Acacia senegal 0.003% DMT, in the leaf (Wahba Khalil & Elkheir 1975 ref Trout's Notes)
Acacia simplexDMT and NMT, in the leaf, stem and trunk bark, 0.81% DMT in bark, MMT
Acacia tortilis Erroneously reported (Trout's Notes)
Acacia sieberiana Erroneously reported (Trout's Notes)
Acacia victorae Aerial parts of 1 year old seed grown material (unconfirmed), Good banding (J Appleseed 1995 ref Trout's notes)
Anadenanthera spp
- Conflicting reports. Most accounts only found bufotenine in seeds, but some reports claim presence of 5-MeO-DMT and/or DMT (Trout's Notes)
- (Argentina) - Snuff believed to be derived from A. colubrina was found with all 3 aforementioned alkaloids but its not clear (Torres et al 1991 ref Trout's Notes)
- (Argentina) - DMT was main or sole alkaloid in pods (Iacobucci & Rúveda 1964 ref Trout's Notes)
- (Argentina) - Only bufotenine in seeds (Trout's Notes)
- (Florida and Brazil) Not detected in seeds, detected in pods as sole alkaloid. Florida material weaker. 1.5-2% total alkaloids in seeds, pods weaker but only DMT found (Fish et al 1955 ref Trout's Notes)
- var Cebil (Argentina) 0.06% in seeds from Misión Wichi and 0.05% in pods from Salta but not detected in seeds from Salta. Traces detected in bark from Cerro San Bernardo (Torres & Repke 1996 ref Trout's Notes)
- DMT in seedpods (sole alkaloid present) (Iacobucci & Rúveda 1964 ref Trout's Notes)
- Seed pods contain dimethyltryptamine and the seeds bufotenin, bufotenin oxide, and oxide of dimethyltryptamine (GRANIER-DOYEUX 1965)
- (Boa Vista, Brazil)DMT at 0.0004% dry bark (1% of total alkaloids), 0.0059% dry leaves (49% of total alkaloids), plus 5-MeO-DMT at 0.025% dry bark and 0.006% dry leaves (Agurell et al 1969 ref Trout's Notes)
Delosperma
(all Delosperma are TLC assays by Appleseed, ref Trout's notes)
- DMT present in undetermined amount. 5 positive assays over a 15 month period. (Xanthydrol-1 and Ehrlich 's-4) (Sept.,
Nov, Dec.) ( 1993-5) Not observed in May assay.
- Sept., Nov. and Dec. assays. 4 positives for DMT over a 25 month period. (Xanthydrol-2 and Ehrlich's-2) (Sasha was unable to confirm this using GC-MS on material purchased from Home Depot in Spring.)
- DMT positive Nov. 1994, 1995 (2, one year apart) also Sept. 1996 (1-Ehrlich's and 2- Xanthydrol)
- DMT - Nov. 1995 assay. Faint (Xanthydrol)
- (Yemen) - Nov. 1995 assay. Faint (Xanthydrol)
- Nov. 1995 and Dec. 1994 assays. Weak DMT band. (Xanthydrol and Ehrlich's)
- Sept. 1996 assay. (Xanthydrol) Co-occurrence with 5-MeO.
- DMT, 5-MEO-DMT (Trout's Notes)
- Dec. 1994 (Ehrl ichs) and August 1995 harvest. Good DMT band (co-occurring with 5-MeO-DMT) (Dec. 1994 harvest; same material retested with Xanthydrol in 1996) Co-occurrence also observed in August and December 1995 harvests assayed in Sept 1996 (Xanthydrol)
- Nov. 1995 assay faint (not present in May assay ) (Xanthydrol) Sept 1996 assay decent. Xanthydrol. No alkaloid observed in Sept 1996 D. pergamentaceum Rooilepel.
- Nov. and Dec. 1994 assay Faint (or was it 5-MeO-DMT'?) (Ehrlich's)
Desmanthus
- 0.34% in Root bark {dried) and 0.01 % in Root wood (dried) (Thompson et al. 1987) Substantially Jess is usually encoun-
tered. Sometimes none. (Trout's Notes)
- 0.14% yield of alkaloid. Identified by Johnny Appleseed 1992. TLC also tested positive 1993- 1995.
- Isolated and Bioassayed as pharmahoasca by J. Appleseed on 28 Nov., 1992.
- Isolated from Central Texas material and bioassayed as partially crystalline free base. Identity confirmed in bioassays by others, 1994 (ref Trout's Notes
- some tested +/ more tested - TLC by J. Appleseed ,1992 (ref Trout's Notes)
Desmodium
- Roots - Major alkaloid 0.087% by dry weight. Ed .: Procedure likely resulted in some loss. If all of their crude alkaloid and all of their picrate had been used they would have obtained 1.46 gm from 1.6 kg dry roots. i.e. - 50 gm of roots for a 45 mg equivalency. Co-occuring with Bufotenine N-Oxide as minor root alkaloid (0.03%; 496 mg from 1.6 kg) (Ueno et at. 1978 ref Trout's Notes)
- Stem - DMT was minor alkaloid 0.0035%; 380 mg from 10.75 gm of stems). Co-occurring with Bufotenine, the major alkaloid in stem (0.04% by dry weight; If they used all of their picrate they would have recovered 4.3 gm of bufotenine base from 10.75 kg of stems.), plus bufotenine n-oxide (0.004%; 447 mg from 10.75 kg of stems. (Ueno et al. 1978 ref Trout's Notes)
- Aerial parts [? gm. of thick oil..:.. 0.41 grm DMT (latter as chloroform soluble acetate) obtained from 1 kg of fresh wet material. (Banerjee & Ghosal 1969 ref Trout's Notes)
- Green Plant (Stem and Leaf) Ghosal 1972a and Ghosal & Bhattacharya 1972; Green material bas 3X more alkaloid than if dried.
- Roots 0.38 gm DMT. from 1.6 kg. of dried roots. i.e.0.02% DMT (Ghosal & Banerjee 1969 ref Trout's Notes))
- Seeds - amount not given (Ghosal & Bhattacharya 1972 ref Trout's Notes)
- Fruit - amount not given (Ghosal 1972a ref Trout's Notes)
- Leaves ( 0.004% in dry leaf: 82 mg from 2 kg.) Ghosal et al. 1972a
- Roots (Minor alkaloid) Ghosal et al. 1972a
- Whole plant (DMT as minor alkaloid) Ghosal & Mukherjee 1964
- Stem and leaf of young seedling - 0.074% DMT by dry weight; 62% of 0.12% Total alkaloid (Ghosal et al 1972c ref Trout's Notes)
- Stem and leaf of mature plant - 0.294% DMT by dry weight; 21% of 1.4% Total alkaloid (Ghosal et al. 1972c ref Trout's Notes)
- Root of young seedling - 0.27% dry weight; 73% of 0.37% Total alkaloid - (Ghosal et al. 1972c ref Trout's Notes)
- Root of mature plant - 0.451% by dry weight; 41 % of 1.1% Total alkaloid - [Also, in same paper: 1.8 kg dried roots yielded 0.7g + 0.09 gm; i.e. 0.043%. (Ghosal et al. 1972c)
- Fruit (green) of mature plant - 12% of 0.01% Total alkaloid; ~0.001% by dry weight - (Ghosal et al. 1972c ref Trout's Notes)
- Seeds (ripe) of mature plant - 4% of 0.02% Total alkaloid; 0.001% by dry weight - (Ghosal et al. 1972c ref Trout's Notes)
- Root, stem-leaf and fruit - Amounts not given - (Ghosal 1972a ref Trout's Notes)
- DMT-N-oxide, roots (Ott)
- Positive TLC assays in seeds, seed pods, stem-bark and roots. (Appleseed ref Trout's Notes)
- Seeds/seed-pods showed same alkaloids as stem-bark but darker and with 3-7 additional bands. (Seeds & pods harvested summer 1994) August stem-bark showed light band. (Appleseed ref Trout's Notes)
- Successful bioassay of 30 gm of red fall leaves reported by Wyrm; pers. comm. (ref Trout's Notes)
Roots harvested in December showed a positive for DMT and lighter for two other bands. (Appleseed 94-95, ref Trout's Notes) Some of these results used Ehrlichs spray and there may be confusion with 5-MeO-DMT in seeds and seeds/seed-pods.
- var. japonica:
- DMT in plant. (Goto et al. 1958 ref Trout's Notes)
- Major alkaloid in leaf and one of the main alkaloids in the root bark. Root bark showed higher concentration than leaves. (Morimoto & Matsumoto 1966 ref Trout's Notes)
- In leaf. (Morimoto & Oshio 1965 ref Trout's Notes)
Diplopterys
- DMT, traces of bufotenine (Mckenna, 1984)
- Leaves - 467mg DMT per 100g dry leaves, co occuring with trace amounts of NMT, Bufotenine, 5-MeO-DMT and MTHBC (Agurell et al 1968)
- Leaves - DMT as only significant peak (Endlessness 2011)
- Stems - 166mg DMT per 100g dry stems, co-occurring with 3mg 5-MeO-DMT and 3mg MTHBC per 100g (Agurel et al 1968)
Justicia spp.
Justicia pectoralis
- In leaf (Shulgin & Shulgin 1997)
- var stenophylla DMT in leaf (Schultes & Holmstedt 1968 ref Trout's Notes). but Mckenna et al 1984 was unable to confirm. TLC bands corresponding to DMT, NMT and another high Rf alkaloid (Appleseed ref Trout's notes)
Grasses
- 20mg from 200grams of dried plant (compared to 520mg gramine per 200g plant!) (Ghosal et al 1971 ref Trout's Notes)
40 mg per 700g rhizome (Dutta & Ghosal 1967 ref Trout's Notes)
- Plants analyzed in india were found with alkaloids, others from USA where not found to contain DMT based alkaloids (Trout notes)
- Numerous essays did not reveal DMT, but other indolic alkaloids (Appleseed & Trout ref Trout's notes)
- Root contains DMT - 0.200% (Ott)Root Bark contains DMT - 0.340% (Ott)
Phalaris aquatica syn. Phalaris tuberosa
- DMT is present in some clones and varieties. DMT in leaf (Baxter & Slaytor 1972; Culvenor et al 1964; Frahn & Illman 1973; Moore et al 1967; Mulvena & Slaytor 1982; Oram & Williams 1967 ref Trout's Notes)
- Clone R16 "Large" amount of DMT co-occuring with "trace" amount of 2MTHBC (Trout's Notes)
- Clone R36 "Trace" amount of DMT co-ocurring with "large" amounts of 2MTHBC (Trout's Notes)
- AQ1 - Highest DMT content in any Phalaris, 1% from grass grown in Italy (Festi & Samorini 1994 ref Trout's notes)
- Commercial var - Weak occurence reported by HPLC (Festi & Samorini 1994b ref Trout's notes)
- Australian Commercial - DMT 280nmol/100 seedlings ( 5-MeO-DMT 150nmol/100 seedlings) , 0.1% DMT dry weight of mature leaf (0.05% 5-MeO-DMT) co-occuring with traces of 5-MeO-T, 5-MeO-NMT (Mulvena & Slaytor 1983 ref Trout's Notes)
- GB 81 - Major base (Frahn & O'Keefe 1971 ref Trout's Notes)
- "High Alkaloid" - Major base (Frahn & O'Keefe 1971)
- JLF - Major base, 5-MeO-DMT & DMT in leaf sept 1995 TLC assay (J Appleseed 1995 ref Trout's Notes)
- Killer - DMT was predominant alkaloid in fall 1994, 5-MeO-DMT was predominant in summer/fall 1995 (J Appleseed 1995 ref Trout's Notes)
- Seedmaster - Major base (Frahn & O'Keefe 1971 ref Trout's Notes)
- Sirocco - 24 nmol of DMT per 100 seedlings (Mulvena & Slaytor 1983)
- DMT is present in some strains but NOT in most (Trout's Notes)
- (France) - Occurrence reported by HPLC (Festi & Samorini 1994b ref Trout's Notes)
- Ottawa Synthetic - Amounts not given, detected by TLC only in some of the samples (Wood & Clark 1971 ref Trout's Notes)
- (Portugal) - Extremely strong occurrence reported, sole alkaloid (Festi & Samorini 1994b ref Trout's Notes)
- (Algeria and greece clones) - Positive human bioassays (Dekorne 1997 ref Trout's Notes)
- USDA PI 202676 and 231044 - No detection, 5-MeO-DMT found instead (J Appleseed ref Trout's Notes)
- (Portugal) - Occurrence reported by HPLC (Festi & Samorini 1994b ref Trout's Notes)
- USDA PI 415833 - Occurrence reported by tlc (Appleseed ref Trout's Notes)
- USDA PI 284185 - Lower levels occurrence reported by tlc (Appleseed ref Trout's Notes)
- (Portugal) - Traces reported by TLC (Festi & Samorini 1994b ref Trout's Notes)
- (Romania) - Occurrence reported by TLC (Festi & Samorini 1994b ref Trout's Notes)
Phalaris stenoptera (= P. aquatica var. stenoptera)
- Variable amounts,Festi & Samorini 1994a cited Rendig et al 1970 as finding 0-60ug/ml of expressed juice (ref Trout's Notes)
- Syn Phalaris aquatica, read above
Leaves and seedlings contain DMT, 5-MeO-DMT, and related compounds (Smith 1977)DMT - 0.100% (erowid)5-MeO-DMT - 0.022% (erowid)5-OH-DMT - 0.005% (erowid)
Mimosa
- Most common commercially available plant source for DMT, getting more controlled and being held in customs of some countries.
- 0.8-2% alkaloids from rootbark (several DMT extractions in dmt-nexus)
- 0.6% DMT (Ref Trout's notes)
- 1.6% DMT in rootbark, co-occuring with NMT (0.0012%), and hordenine (0.0065%) (Batista et al 1999)
- DMT in bark (Ott)
- DMT (Schultes 1969)
Mucuna spp
- Leaves, seeds, stems and roots contain L-Dopa, Serotonin, 5-HTP, and Nicotine, as well as N,N-DMT, Bufotenine, and 5-MeO-DMT (Erowid)
Osteophloem spp
- DMT, 5-MEO-DMT in bark (Ott)
Petalostylis
- 0.4-0.5% tryptamine, DMT, etc. in leaves and stems (Johns et al 1966)
Petalostylys spp
Petalostylis labicheoidesvar. casseoides
- DMT in leaves and stems (Ott)
Psychotria spp.
- 0.2% average DMT in dried leaves (Ott)
Virola
- Leaves 0.149% DMT (Ott)
- DMT in leaves (Ott)
- DMT in leaves (Ott)
- DMT, 5-MEO-DMT in bark and leaves (Ott)
- DMT in bark (Ott)
- DMT, 5-MEO-DMT in bark and leaves (Ott)
- DMT in leaves (Ott)
- DMT, 5-MEO-DMT in bark (Ott)
- Alkaloids in bark and root, 95% of which is 5-MeO-DMT (Shulgin, TIHKAL)
- DMT in bark (Ott)
- DMT, 5-MEO-DMT in bark, roots, leaves and flowers (Ott)
- DMT, 5-MEO-DMT in roots and leaves (Ott)
Extraction Teks
For an overview on how extractions work, read the FAQ, and the Extraction Overview
A/B
STB
STB-A/B hybrid
Dry tek
Dosages and consumption methods
Smoked / Vaporized
Extracted DMT freebase can be vaporized for very potent effects that last around 10 minutes. DMT is ideally vaporized, as opposed to smoked. Vaporization is achieved by a controlled temperature that does not burn/combust DMT material (and potential impurities), but instead just makes DMT evaporate and be inhaled.
Vaporization is much smoother than smoking. Smoking leads to break down of DMT (and impurity) molecules into potential toxic nitrogen oxides (Trout's notes), so not only it is harsher but also there is a significant loss of actives.
Vaporizing can be achieved with improvised vaporizers such as The Inspirator mkII, or commercially sold vaporizing pipes such as the VaporGenie.
Some methods, such as The Machine, if it's very carefully done, keeping the lighter farther away, one can also vaporize DMT, but due to lack of adequate buffer between the fire and the alkaloids, often will also generate combustion.
Smoking is nonetheless still a popular way of ingesting DMT, and is often done by infusing herbs with the DMT, or smoking in a bong, with the DMT sandwhiched between thick layer of ashes or thin layer of herbs that serve to protect the DMT from fire (though there is still combustion, specially when using herbs).
Dosages are around 20-30mg for efficient vaporization methods, and with smoking methods can be around 50-60mg or even more....
Oral
DMT is only active orally when taking together with a MAOI. (FAQ for more info)
Dosages for DMT, considering MAOs are fully inhibited, vary wildly depending on person, probably due to metabolism in great part. They can go from 30 to 250mg! If its your first time, start on the lower end!
Another factor is whether one is ingesting a whole plant brew or purified extracts. Often in ayahuasca analysis the amount of DMT found is very small (20-30mg), but also often there is redosing in ayahuasca sessions, but also its possible other trace amount of beta-carbolines and alkaloids can improve MAO inhibition, or that other inactive plant substances can help protecting DMT from fast breakdown by any potential active MAO.
There are a few different ways to ingest it orally:
- Dissolved in acidic juice - Rolled inside a bit of smoking paper and swallowed like a pill - Put into 00 Capsules
Snorted
This is a method that gets very opposite responses from different people. For many, it hurts too much and isn't effective. For others it works well and pain/discomfort is tolerable, and the effects are worth it. It is unknown what possible health consequences snorting a basic alkaloid such as DMT can have on nasal passages, specially long term use, so we advice caution. There are some attempts to find less harsh ways, check threads below for more info:
Snorting Works! Preparations to make snorting more tolerable
History of usage
Analysis of DMT
To learn how analytical processes work, follow this link
Colorimetric reagents
References here
DMT
- α-Nitroso-β-naphthol-nitrous acid - Negative - (silica gel) - (23)
- - Weak brown (on paper) - (18 )
- Chloranil - No Reaction - (silica gel) - ( 8 )
- CNTF - Gray (light) - (silica gel) - ( 8 )
- Diazotized p-Nitroaniline - Very weak yellow - (on paper) - (18 )
- Dragendorff's - positive with spray - (silical gel) - (5)
- Red-Brown - (paper) - (18 ) - Orange - (silica gel) - (23)
- Ehrlich - Reddish purple - (as acetate on paper) - (26)
- Fluoranil - Purple - (silica gel) - ( 8 )
- Fluorescence with PENE - Violet under 254nm UV - (silica gel with PENE) - (24)
- HNS - No reaction - (silica gel) - ( 8 )
- HNO3 atmosphere - yellow - (silica gel) - (25)
- Iodine vapor - Red-Brown - (paper) - (18 )
- Iodoplatinate - Purple - (silica gel) - (25)
- Blue (silica gel) - (17)
- Iodoplatinate, acidified - Positive - (silica gel) - (5)
- Marquis - Yellow - (NA) - (13)
- Marquis - GreenYellow - (silica gel) - (7)
- Marquis - Orange->red - (NA) - (5)
- Mecke - Brown->red over time time. - (NA) - (13)
- Mandellin - yellow - (NA) - (13)
- Ninhydrin, acetic acid - No UV fluorescence - (acetate on paper) - (26)
- No visible color - (acetate on paper) - (26)
- NNCD - Weak orange - (on paper) - (19)
- p-DMAB, ethanol:sulphuric - Red solution, Violet when diluted with water - (5)
- p-DMAB-TS - Yellow - (pure compound) - (3, 27)
- p-DMAB, ethanolic - Purple - (pure compound) - (3)
- TACOT - Purple (light) - (silica gel) - ( 8 )
- TCBI - Brown-green - (silica gel) - ( 8 )
- TCNE - Brown (light and fading) - (silica gel) - ( 8 )
- TetNF - Brown (light) - (silica gel) - ( 8 )
- TNB - Yellow->Brown - (silica gel) - ( 8 )
- TNF - Brown (light) - (silica gel) - ( 8 )
- Van Urk - Blue - (silica treated with 0.1M KOH) - (16)
- Xanthydrol - Purple - (silica gel & celulose) - (15)
- Purple - (tlc & on paper) - (5, 20) - Pink - (on paper) - (21) - Lavender - (on paper) - (22)
LC / GC-MS
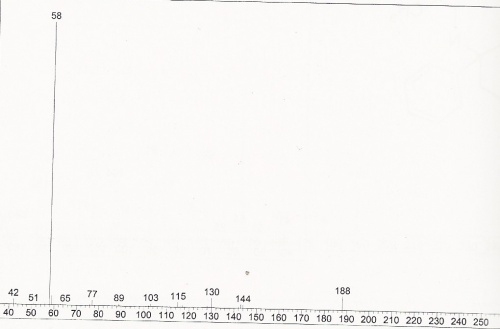
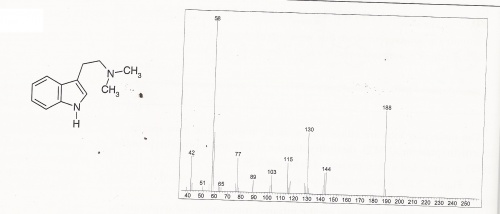
DMT Mass Spectra
DMT Mass Spectra (expanded)
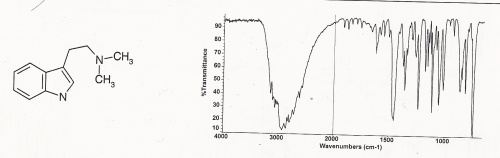
IR - Infrared
Other IR data (Clarke's second): Principal peaks at wavenumbers 743, 1113, 1235, 1050, 812, 1010 (KBr disk)
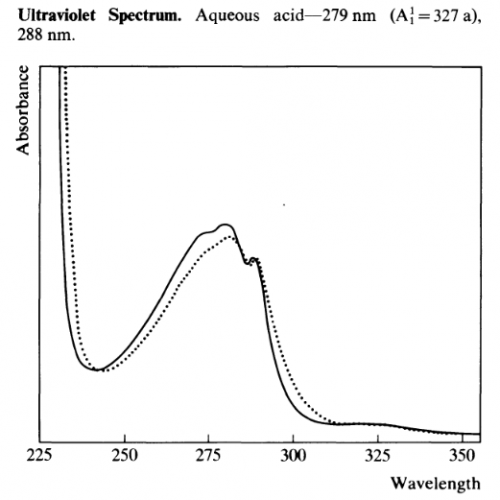
UV-Vis
Other UV-Vis data:
- λmax 222nm (log e 4.48), 277 (3.77) and 288 (3.75) Ghosal et al 1969
- λmax 222-224, 274 & 294nm Banergee & Ghosal 1969
- λmax 222, 277, 287 & 294 nm Ghosal & Banergee 1969
- λmax 274, 283, 291nm (refernce material) 275, 283, 291nm (isolated material) Fish et al 1955
- λmax (CH3OH): 220, 280, 290 (= 5500, 5600, 5000) De Moraes et al 1990
- λmax (EtOH): 226, 275 (sh), 279, 284, 293nm Grina et al 1982
- λmax 276, 282, 290nm
λmin 278, 287nm Martin & Alexander 1968
- λmax 275, 219 (0.1N NaOH)
- λmax 290, 276, 282 (EtOH) Sunshine 1981
- λmax of Xanthydrol reactive product (CHCl3): 510nm
λmin of Xanthydrol reactive product (CHCl3): 400nm Gander et al 1976
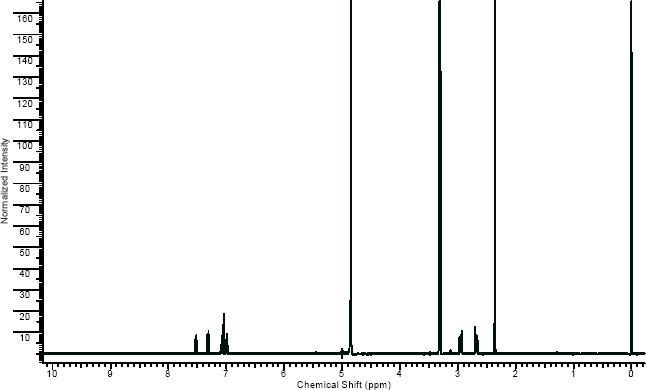
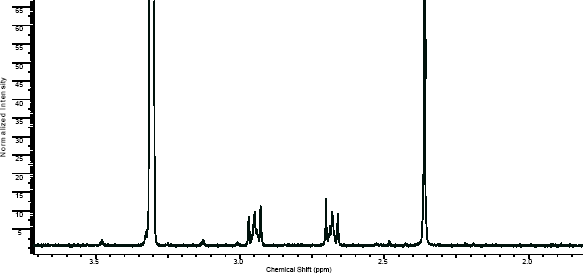
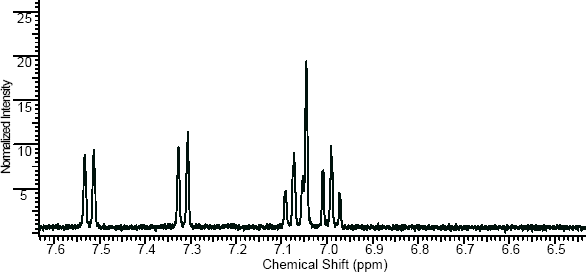
NMR
(NMR info source, method description and results discussion:Microgram bulletin volume 5, n14, pg6 )
Scientific publications
Links of interest
Pages in category "DMT"
The following 40 pages are in this category, out of 40 total.