Difference between revisions of "Category:DMT"
TheTraveler (Talk | contribs) m |
Endlessness (Talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | == What | + | http://wiki.dmt-nexus.me/Category:Alkaloids |
| + | |||
| + | == Brief overview - What is DMT? == | ||
NN-Dimethyltryptamine or DMT for short is an short acting psychedelic entheogen which allows a persons consciousness to voyage into the most incredible dimensions, visions, thoughts and experiences imaginable. | NN-Dimethyltryptamine or DMT for short is an short acting psychedelic entheogen which allows a persons consciousness to voyage into the most incredible dimensions, visions, thoughts and experiences imaginable. | ||
| Line 8: | Line 10: | ||
DMT is closely related to serotonin, the naturally occurring neurotransmitter that psychedelics affect so widely. The pharmacology of DMT is similar to that of other well-known psychedelics. It affects receptor sites for serotonin in much the same way that LSD, psilocybin, and mescaline do. These serotonin receptors are widespread throughout the body and can be found in blood vessels, muscle, glands, and skin. | DMT is closely related to serotonin, the naturally occurring neurotransmitter that psychedelics affect so widely. The pharmacology of DMT is similar to that of other well-known psychedelics. It affects receptor sites for serotonin in much the same way that LSD, psilocybin, and mescaline do. These serotonin receptors are widespread throughout the body and can be found in blood vessels, muscle, glands, and skin. | ||
| + | |||
| + | == Chemical and physical properties == | ||
| + | |||
| + | === DMT === | ||
| + | N,N-Dimethyltryptamine | ||
| + | |||
| + | ==== Freebase DMT ==== | ||
| + | <table><tr><td>[[Image:dmtfreebase.png]]</td><td></table> | ||
| + | * Appearance: White/Transparent crystals | ||
| + | * CAS Registry Number: 61-50-7 | ||
| + | * Composition: C12H16N2 | ||
| + | * Molecular Weight: 188.26884 g/mol | ||
| + | * Melting point: 44-68°C (Conflicting reports in literature, as mentioned in [http://isomerdesign.com/PiHKAL/browse.php?domain=tk TIHKAL]) | ||
| + | * Boiling point: Theoretically 360-380°C | ||
| + | * XLogP: 2.0 | ||
| + | * XLogP3: 2.5 ([http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6089&loc=ec_rcs PubChem]) | ||
| + | * pKa: 8.68 ([http://wiki.dmt-nexus.com/w/images/8/81/MERCK.pdf Merck Index]) | ||
| + | * Colorimetric reagent results: [https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=25771 Here] | ||
| + | * Stability/Degradation: Oxidation to DMT N-Oxide (yellow oil) in extended presence of oxygen (specialy in evaporation of dmt-containing solvents with heat and/or fan or generally in prolonged exposure to open air). N-oxide may be reverted back to the parent compound by reduction, as described [http://www.anoniem.org/?http://wiki.dmt-nexus.com/DMT_N-Oxide_to_Freebase_DMT in the N-Oxide to Freebase Wiki]. | ||
| + | * Solubility: | ||
| + | Very Soluble in Xylene, Toluene, Limonene, acetone, Isopropyl Alcohol (IPA), methanol, ethanol, Dichloromethane (DCM), chloroform, ether, Butanone (also known as methyl ethyl ketone (MEK)) and butanol. | ||
| + | |||
| + | Soluble in naphtha, hexane, heptane but almost insoluble in these solvents at freezing temperatures | ||
| + | |||
| + | Almost insoluble in water. | ||
| + | |||
| + | ==== DMT N-Oxide ==== | ||
| + | <table><tr><td>[[Image:dmtnoxide.jpg]]</td><td></table> | ||
| + | * Appearance: Yellow oil | ||
| + | * XLogP3: 2 | ||
| + | * Colorimetric reagent results: [https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=25771 Here] | ||
| + | * Solubility: | ||
| + | Soluble in Xylene, Toluene, Limonene | ||
| + | |||
| + | Slightly soluble in basic water | ||
| + | |||
| + | Insoluble in naphtha | ||
| + | |||
| + | ==== DMT Fumarate ==== | ||
| + | |||
| + | * Molecular Weight: 492.608 g/mol | ||
| + | * Solubility: | ||
| + | Very soluble in water | ||
| + | |||
| + | Soluble in methanol (~10mg/ml) | ||
| + | |||
| + | Soluble in boiling IPA, Practically insoluble in room temp IPA (~1mg/ml), Insoluble in freeze-cold IPA. | ||
| + | |||
| + | Slightly soluble in ethanol (~5mg/ml) | ||
| + | |||
| + | Insoluble in cold acetone | ||
| + | |||
| + | Insoluble in FASI (Fumaric Acid Saturated IPA) | ||
| + | |||
| + | Insoluble in FASA (Fumaric Acid Saturated Acetone) | ||
| + | |||
| + | |||
| + | == Effects == | ||
| + | Depending on the dosage, the effects of DMT can range from a multitude of sensations, from bizarre, beautiful and even 'impossible' visions to literally jaw hanging awe as one is propelled into other dimensions of existence where human language and logic cannot even begin to describe or comprehend. | ||
| + | |||
| + | There have been a few attempts to define different levels and types of experience. Psychedelic Monographs and Essays<ref>Psychedelic Monographs and Essays | ||
| + | [http://www.erowid.org/library/books/psychedelic_monographs_5.shtml]</ref> discusses different levels of a DMT experience. | ||
| + | |||
| + | The [[Hyperspace Lexicon]] project is an attempt to create a new vocabulary to try to describe the DMT realm | ||
| + | |||
| + | |||
| + | == Plants containing DMT == | ||
| + | |||
| + | The following is a list of plants known to contain tryptamines. | ||
| + | |||
| + | === Acacia === | ||
| + | {{:Acacia_acuminata}} | ||
| + | |||
| + | {{:Acacia_alpina}} | ||
| + | |||
| + | {{:Acacia angustifolia}} | ||
| + | |||
| + | {{:Acacia angustissima}} | ||
| + | |||
| + | {{:Acacia auriculiformis}} | ||
| + | |||
| + | {{:Acacia baileyana}} | ||
| + | |||
| + | {{:Acacia berlandieri}} | ||
| + | |||
| + | {{:Acacia catechu}} | ||
| + | |||
| + | {{:Acacia caven}} | ||
| + | |||
| + | {{:Acacia colei}} | ||
| + | |||
| + | {{:Acacia complanata}} | ||
| + | |||
| + | {{:Acacia constricta}} | ||
| + | |||
| + | {{:Acacia confusa}} | ||
| + | |||
| + | {{:Acacia cornigera}} | ||
| + | |||
| + | {{:Acacia cultriformis}} | ||
| + | |||
| + | {{:Acacia farnesiana}} | ||
| + | |||
| + | {{:Acacia filiciana}} | ||
| + | |||
| + | {{:Acacia_floribunda}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia georginae| | ||
| + | * Psychoactive,[8] plus deadly toxins | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia greggii| | ||
| + | * N-methyl-β-phenethylamine,[12] phenethylamine[36] | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia harpophylla| | ||
| + | * Phenethylamine, hordenine at a ratio of 2:3 in dried leaves, 0.6% total[6] | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia holoserica| | ||
| + | * Hordenine, 1.2% in bark[6] | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia horrida| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia implexa| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia karroo| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia kempeana| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia kettlewelliae| | ||
| + | * 1.5[6]–1.88%[38] alkaloids, 92% consisting of phenylethylamine.[6] 0.9% N-methyl-2-phenylethylamine found a different time | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia laeta| | ||
| + | * DMT in the leaf (Trout's Notes) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia lingulata| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia longifolia| | ||
| + | * 0.2% tryptamine in bark, leaves, some in flowers, phenylethylamine in flowers (Hegnauer 1994) | ||
| + | * DMT in plant (Lyceaum) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia macradenia| | ||
| + | * Tryptamine | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia maidenii| | ||
| + | * Bark of A. maidenii contains 0.6% of N-methyltryptamine and DMT in the proportions approx. 2:3 (Fitzgerald & Sioumis 1965) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia mangium| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia melanoxylon| | ||
| + | * DMT in the bark and leaf, less than 0.02% total alkaloids (Hegnauer 1994) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia mellifera| | ||
| + | * DMT in the leaf (Trout's Notes) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia nilotica| | ||
| + | * DMT in the leaf (Trout's Notes) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia obtusifolia| | ||
| + | * 0.4 to 0.5 % DMT/NMT in the dried bark (Csiro 1990) | ||
| + | *0.15-0.6% DMT,NMT(2:1)plus trace betacarboline in bark, 0.06-0.2% leaves (Southern Cross University comissioned test 2001) | ||
| + | *5-MeoDMT & bufotenine in some loctations (E., Entheogen Review 1995-6; Trout's Notes 2005-10) Is not fast growing in the wild and is under threat of serious overharvesting. Is NOT considered a weed as previously stated here, and will become rarer if wild seed populations exploited further.(Nen, original bioassay subject) | ||
| + | {{botanics_info|image:NoImage.png|Acacia oerfota| | ||
| + | * Less than 0.1% DMT in leaf (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia penninervis| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia phlebophylla| | ||
| + | * 0.3% DMT in leaf, NMT (Trout's Notes) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia platensis| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia podalyriaefolia| | ||
| + | * Tryptamine in the leaf (Trout's Notes) | ||
| + | * 0.5% to 2% DMT in fresh bark, phenethylamine trace amounts (Hegnauer 1994) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia polyacantha| | ||
| + | * DMT in leaf (Trout's Notes) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia pycantha| | ||
| + | * Psychoactive,[8] but less than 0.02% total alkaloids | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia retinodes| | ||
| + | * DMT and MMT (www.factorey.ch/Eins.htm) | ||
| + | * Less than 0.02% total alkaloids found (Hegnauer 1994) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia roemeriana| | ||
| + | * β-methyl-phenethylamine | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia rigidula| | ||
| + | * DMT, NMT, tryptamine, amphetamines, mescaline, nicotine and others (Phytochem. 199 | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia salicina| | ||
| + | * Psychoactive[8][9] Ash used in Pituri. | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia sassa| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia schaffneri| | ||
| + | * β-methyl-phenethylamine, Phenethylamine[36] Amphetamines and mescaline also found | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia schottii| | ||
| + | * β-methyl-phenethylamine | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia senegal| | ||
| + | * DMT, in the leaf | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia simplex| | ||
| + | *DMT and NMT, in the leaf, stem and trunk bark, 0.81% DMT in bark, MMT | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia taxensis| | ||
| + | * β-methyl-phenethylamine | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia tenuifolia| | ||
| + | * Psychoactive | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|File:290px-Eat267.jpg|Acacia tortilis| | ||
| + | * DMT, NMT, and other tryptamines | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia sieberiana| | ||
| + | * DMT in the leaf (Trout's Notes) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia verek| | ||
| + | * Psychoactive (Rätsch 2004) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Acacia vestita| | ||
| + | * Tryptamine, in the leaf and stem (Trout's Notes) | ||
| + | * Less than 0.02% total alkaloids (Hegnauer 1994) | ||
| + | }} | ||
| + | |||
| + | === Anadenanthera === | ||
| + | {{:Anadenanthera colubrina}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Anadenanthera macrocarpa| | ||
| + | * Seed pods contain dimethyltryptamine and the seeds bufotenin, bufotenin oxide, and oxide of dimethyltryptamine (GRANIER-DOYEUX 1965) | ||
| + | }} | ||
| + | |||
| + | {{:Anadenanthera_peregrina}} | ||
| + | |||
| + | === Common Reed === | ||
| + | {{botanics_info|image:arundo_donax.jpg|Arundo donax|Entire Plant contains 5-MeO-DMT (Shulgin, TIHKAL)Flowers contain DMT, 5-MeO-DMT, and 5-MeO-NMT (Shulgin, TIHKAL)Roots contain DMT, 5-MeO-DMT, 5-MeO-NMT, Bufotenine, bufotenidine, dehydrobufotenidine (Shulgin, TIHKAL)}} | ||
| + | |||
| + | {{botanics_info|image:desmanthus.jpg|Desmanthus illinoensis|Root contains DMT - 0.200% (Ott)Root Bark contains DMT - 0.340% (Ott)}} | ||
| + | |||
| + | {{:Phalaris_arundinacea}} | ||
| + | |||
| + | {{botanics_info|image:phalaris_aquatica.jpg|Phalaris tuberosa|Leaves and seedlings contain DMT, 5-MeO-DMT, and related compounds (Smith 1977)DMT - 0.100% (erowid)5-MeO-DMT - 0.022% (erowid)5-OH-DMT - 0.005% (erowid)}} | ||
| + | |||
| + | {{:Phragmites_australis}} | ||
| + | |||
| + | === Delosperma === | ||
| + | {{botanics_info|Image:Delosperma acuminatum2.jpg|Delosperma acuminatum|DMT, 5-MEO-DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:delosperma_acuminatum.jpg|Delosperma cooperi|DMT, 5-MEO-DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:iceplantbrighteyes-may.jpg|Delosperma ecklonis|DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:Delosperma_esterhuyseniae.jpg|Delosperma esterhuyseniae|DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Delosperma hallii|5-MEO-DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Delosperma harazianum|DMT, 5-MEO-DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Delosperma hirtum|DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:DelospermaLydenbergense.jpg|Delosperma lydenbergense|DMT, 5-MEO-DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Delosperma nubigenum|5-MEO-DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Delosperma pageanum|DMT, 5-MEO-DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Delosperma pergamentaceum|Traces of DMT (Trout's Notes)}} | ||
| + | |||
| + | {{botanics_info|image:180px-Delosperma_tradescantioides_leafs_IMGP0042.jpg|Delosperma tradescantioides|DMT (Trout's Notes)}} | ||
| + | |||
| + | === Desmodium === | ||
| + | {{botanics_info|image:NoImage.png|Desmodium caudatum| | ||
| + | * Roots: 0.087% DMT, Bufotenine-N-oxide 0.03% (Trout's Notes) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Desmodium gangeticum| | ||
| + | * DMT, 5-MEO-DMT, whole plant, roots, stems, leaves (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Desmodium gyrans| | ||
| + | * DMT, 5-MEO-DMT, leaves, roots (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Desmodium pulchellum| | ||
| + | * DMT, 5-MEO-DMT, whole plant, roots, stems, leaves, flowers (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Desmodium racemosum| | ||
| + | * 5-MEO-DMT (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Desmodium triflorum| | ||
| + | * DMT-N-oxide, roots (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Lespedeza bicolor|var. japonica | ||
| + | * DMT, 5-MEO-DMT in leaves and root bark (Ott) | ||
| + | }} | ||
| + | |||
| + | === Petalostylis === | ||
| + | {{botanics_info|image:petalostylis_cassioides.jpg|Petalostylis cassioides|0.4-0.5% tryptamine, DMT, etc. in leaves and stems (Johns et al 1966)}} | ||
| + | |||
| + | === Mimosa === | ||
| + | {{:Mimosa hostilis}} | ||
| + | |||
| + | {{:Mimosa ophthalmocentra}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Mimosa scabrella| | ||
| + | * DMT in bark (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|File:250px-Mimosa_verrucosa01.jpg|Mimosa verrucosa| | ||
| + | * DMT (Schultes 1969) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Mucuna pruriens| | ||
| + | * Leaves, seeds, stems and roots contain L-Dopa, Serotonin, 5-HTP, and Nicotine, as well as N,N-DMT, Bufotenine, and 5-MeO-DMT (Erowid) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Petalostylis labicheoides|var. casseoides | ||
| + | * DMT in leaves and stems (Ott) | ||
| + | }} | ||
| + | |||
| + | {{:Diplopterys_cabrerana}} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Horsfieldia superba| | ||
| + | * 5-MeO-DMT and beta-carbolines (Jossang et al. 1991) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Iryanthera ulei| | ||
| + | * 5-MEO-DMT in bark (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Osteophloem platyspermum| | ||
| + | * DMT, 5-MEO-DMT in bark (Ott) | ||
| + | }} | ||
| + | |||
| + | === Virola=== | ||
| + | {{botanics_info|image:NoImage.png|Virola calophylla| | ||
| + | * Leaves 0.149% DMT (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola carinata| | ||
| + | * DMT in leaves (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola divergens| | ||
| + | * DMT in leaves (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola elongata| | ||
| + | * DMT, 5-MEO-DMT in bark and leaves (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola melinonii| | ||
| + | * DMT in bark (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola multinervia| | ||
| + | * DMT, 5-MEO-DMT in bark and leaves (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola pavonis| | ||
| + | * DMT in leaves (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola peruviana| | ||
| + | * DMT, 5-MEO-DMT in bark (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola rufuta| | ||
| + | * Alkaloids in bark and root, 95% of which is 5-MeO-DMT (Shulgin, TIHKAL) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola sebifera| | ||
| + | * DMT in bark (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola theiodora| | ||
| + | * DMT, 5-MEO-DMT in bark, roots, leaves and flowers (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Virola venosa| | ||
| + | * DMT, 5-MEO-DMT in roots and leaves (Ott) | ||
| + | }} | ||
| + | |||
| + | {{botanics_info|image:NoImage.png|Psychotria carthaginensis| | ||
| + | * 0.2% average DMT in dried leaves (Ott) | ||
| + | }} | ||
| + | |||
| + | {{:Psychotria_viridis}} | ||
| + | |||
| + | ==Sources== | ||
| + | |||
| + | {{Page_Footer|Botanicals|DMT}} | ||
| + | |||
| + | |||
| + | == Extraction Teks == | ||
| + | ===A/B=== | ||
| + | |||
| + | * [[Lextek]] | ||
| + | * [[Marsofold's tek]] | ||
| + | * [[Shaggy's Jungle Tek]] | ||
| + | * [[The DMT Handbook]] | ||
| + | * [[Vovin's tek]] | ||
| + | |||
| + | ===STB=== | ||
| + | |||
| + | * [[Jorkest's D-Limonene and Fumaric Acid Approach]] | ||
| + | * [[Lazyman's tek]] | ||
| + | * [[Noman's tek]] | ||
| + | |||
| + | ===STB-A/B hybrid=== | ||
| + | |||
| + | * [[BLAB_-_The_Big_Leisurely_A/B|BLAB]] | ||
| + | * [[PanoraMIX European AB]] | ||
| + | * [[Nontoxic_limonene_tek|SyZyGyPSy's Nontoxic Limomene Tek]] | ||
| + | |||
| + | ===Dry tek=== | ||
| + | |||
| + | * [[Amor fati's Nontoxic Approach to Spice Extraction]] | ||
| + | * [[Q21Q21's Vinegar/Lime A/B Extraction Tek]] | ||
| + | |||
| + | == Dosages and consumption methods == | ||
| + | == History of usage == | ||
| + | == Scientific publications == | ||
| + | == Links of interest == | ||
| + | |||
| + | |||
| + | |||
| + | == What Is DMT? == | ||
| + | |||
== What Are The Effects? == | == What Are The Effects? == | ||
Revision as of 17:00, 4 November 2011
http://wiki.dmt-nexus.me/Category:Alkaloids
Contents
- 1 Brief overview - What is DMT?
- 2 Chemical and physical properties
- 3 Effects
- 4 Plants containing DMT
- 5 General Plant Info
- 6 Geographic distribution
- 7 Identification
- 8 Alkaloid content
- 9 Other uses
- 10 Extraction
- 11 Cultivation
- 12 Suppliers
- 13 Links
- 14 References
- 15 General Plant Info
- 16 Identification
- 17 Alkaloid content
- 18 Extraction teks
- 19 Cultivation
- 20 Refinery for the Purpose of Extraction
- 21 References
- 22 Links
- 23 Sources
- 24 Extraction Teks
- 25 Dosages and consumption methods
- 26 History of usage
- 27 Scientific publications
- 28 Links of interest
- 29 What Is DMT?
- 30 What Are The Effects?
- 31 Is DMT Dangerous?
- 32 How Can One Acquire DMT?
- 33 Reference
- 34 Links
Brief overview - What is DMT?
NN-Dimethyltryptamine or DMT for short is an short acting psychedelic entheogen which allows a persons consciousness to voyage into the most incredible dimensions, visions, thoughts and experiences imaginable.
It is without doubt one of the most powerful yet mysterious psychedelics in existence but to classify DMT as merely a drug would be doing it a great injustice as DMT is more a trans dimensional key into places and vistas so profound and awe inspiring that it raises many new questions regarding the nature of reality and our place within it.
DMT exists naturally in every human being and also throughout the plant and animal kingdoms. It occurs naturally in many mammals, marine animals, trees, grasses, flowers and shoots.
DMT is closely related to serotonin, the naturally occurring neurotransmitter that psychedelics affect so widely. The pharmacology of DMT is similar to that of other well-known psychedelics. It affects receptor sites for serotonin in much the same way that LSD, psilocybin, and mescaline do. These serotonin receptors are widespread throughout the body and can be found in blood vessels, muscle, glands, and skin.
Chemical and physical properties
DMT
N,N-Dimethyltryptamine
Freebase DMT
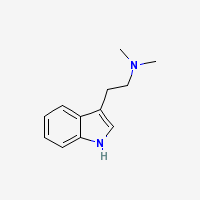 |
- Appearance: White/Transparent crystals
- CAS Registry Number: 61-50-7
- Composition: C12H16N2
- Molecular Weight: 188.26884 g/mol
- Melting point: 44-68°C (Conflicting reports in literature, as mentioned in TIHKAL)
- Boiling point: Theoretically 360-380°C
- XLogP: 2.0
- XLogP3: 2.5 (PubChem)
- pKa: 8.68 (Merck Index)
- Colorimetric reagent results: Here
- Stability/Degradation: Oxidation to DMT N-Oxide (yellow oil) in extended presence of oxygen (specialy in evaporation of dmt-containing solvents with heat and/or fan or generally in prolonged exposure to open air). N-oxide may be reverted back to the parent compound by reduction, as described in the N-Oxide to Freebase Wiki.
- Solubility:
Very Soluble in Xylene, Toluene, Limonene, acetone, Isopropyl Alcohol (IPA), methanol, ethanol, Dichloromethane (DCM), chloroform, ether, Butanone (also known as methyl ethyl ketone (MEK)) and butanol.
Soluble in naphtha, hexane, heptane but almost insoluble in these solvents at freezing temperatures
Almost insoluble in water.
DMT N-Oxide
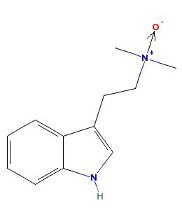 |
- Appearance: Yellow oil
- XLogP3: 2
- Colorimetric reagent results: Here
- Solubility:
Soluble in Xylene, Toluene, Limonene
Slightly soluble in basic water
Insoluble in naphtha
DMT Fumarate
- Molecular Weight: 492.608 g/mol
- Solubility:
Very soluble in water
Soluble in methanol (~10mg/ml)
Soluble in boiling IPA, Practically insoluble in room temp IPA (~1mg/ml), Insoluble in freeze-cold IPA.
Slightly soluble in ethanol (~5mg/ml)
Insoluble in cold acetone
Insoluble in FASI (Fumaric Acid Saturated IPA)
Insoluble in FASA (Fumaric Acid Saturated Acetone)
Effects
Depending on the dosage, the effects of DMT can range from a multitude of sensations, from bizarre, beautiful and even 'impossible' visions to literally jaw hanging awe as one is propelled into other dimensions of existence where human language and logic cannot even begin to describe or comprehend.
There have been a few attempts to define different levels and types of experience. Psychedelic Monographs and Essays[1] discusses different levels of a DMT experience.
The Hyperspace Lexicon project is an attempt to create a new vocabulary to try to describe the DMT realm
Plants containing DMT
The following is a list of plants known to contain tryptamines.
Acacia
| Acacia acuminata |
|
|---|---|
| Up to 1.8% alkaloids, mainly consisting of dimethyltryptamine in bark (Jeremy EGA conference, Australia 2009, used successfully in S.Australia since c.2008); up to 1.2% DMT phyllode (leaf) (DMT-Nexus); tryptamine in leaf (White et al 1951) |
| Acacia alpina |
|
|---|---|
| Active principles (mainly DMT) in leaf (M.Bock) |
| Acacia angustifolia |
|
|---|---|
| Psychoactive Tryptamines (Rätsch 2004) |
| Acacia angustissima |
|
|---|---|
| β-methyl-phenethylamine (Glasby 1991)NMT and DMT in leaf, 1.2-2.8 ppm (McSweeney et al. 2005) |
| Acacia auriculiformis |
|
|---|---|
| 5-MeO-DMT in stem bark (Lycaeum) |
| Acacia baileyana |
|
|---|---|
|
| Acacia berlandieri |
|
|---|---|
|
| Acacia catechu |
|
|---|---|
|
| Acacia caven |
|
|---|---|
|
[[ |center|100x100px]] |center|100x100px]] |
Acacia colei |
|---|---|
| DMT (Dr. Karl and abc.net.au 2005) 1%+ in bark (different net reports) |
</onlyinclude>
General Plant Info
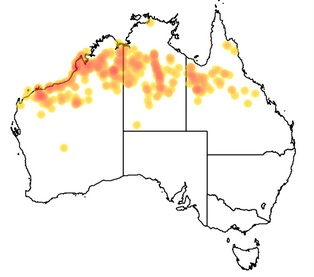
Acacia colei is a perennial bush or tree native to Australia and southern Asia. A common name for it is Cole's Wattle. It grows to a height of up to 9 m. Acacia colei blooms from June through July and the flowers are bright yellow.[2]
Consists of 2 variants:
Geographic distribution
Identification
Phyllodes are 10-19 cm long and 20-55 mm wide, usually with three prominent longitudinal nerves. A dense covering of short hairs on the phyllodes gives the plant a characteristic silvery-blue appearance.
Alkaloid content
Claimed to contain 1.8% or more DMT in bark [3] [4], 0.2-0.6% in leaf. Needs further research.
Other uses
Its uses include environmental management, forage and wood. The seeds are good-tasting[5] and are potentially useful as food for humans. The results of tests in Nigeria for the feasibility of raising the tree as a drought-resistant food crop came out very positively.[6]
Extraction
Cultivation
Suppliers
Links
References
- ↑ Psychedelic Monographs and Essays [1]
- ↑ Australian Biological Resources Study
- ↑ Dr. Karl Kruszelnicki ABC Radio
- ↑ Seldom/nen888 DMT Nexus
- ↑ ECHO Education Concerns for Hunger Organization
- ↑ World Wide Wattle
| Acacia complanata |
|
|---|---|
|
| Acacia constricta |
|
|---|---|
|
| Acacia confusa |
|
|---|---|
|
| Acacia cornigera |
|
|---|---|
|
| Acacia cultriformis |
|
|---|---|
|
| Acacia farnesiana |
|
|---|---|
|
| Acacia filiciana |
|
|---|---|
|
| Acacia floribunda |
|
|---|---|
|
| Acacia georginae |
|
|---|---|
|
| Acacia greggii |
|
|---|---|
|
| Acacia harpophylla |
|
|---|---|
|
| Acacia holoserica |
|
|---|---|
|
| Acacia horrida |
|
|---|---|
|
| Acacia implexa |
|
|---|---|
|
| Acacia karroo |
|
|---|---|
|
| Acacia kempeana |
|
|---|---|
|
| Acacia kettlewelliae |
|
|---|---|
|
| Acacia laeta |
|
|---|---|
|
| Acacia lingulata |
|
|---|---|
|
| Acacia longifolia |
|
|---|---|
|
| Acacia macradenia |
|
|---|---|
|
| Acacia maidenii |
|
|---|---|
|
| Acacia mangium |
|
|---|---|
|
| Acacia melanoxylon |
|
|---|---|
|
| Acacia mellifera |
|
|---|---|
|
| Acacia nilotica |
|
|---|---|
|
{{botanics_info|image:NoImage.png|Acacia obtusifolia|
- 0.4 to 0.5 % DMT/NMT in the dried bark (Csiro 1990)
- 0.15-0.6% DMT,NMT(2:1)plus trace betacarboline in bark, 0.06-0.2% leaves (Southern Cross University comissioned test 2001)
- 5-MeoDMT & bufotenine in some loctations (E., Entheogen Review 1995-6; Trout's Notes 2005-10) Is not fast growing in the wild and is under threat of serious overharvesting. Is NOT considered a weed as previously stated here, and will become rarer if wild seed populations exploited further.(Nen, original bioassay subject)
| Acacia oerfota |
|
|---|---|
|
| Acacia penninervis |
|
|---|---|
|
| Acacia phlebophylla |
|
|---|---|
|
| Acacia platensis |
|
|---|---|
|
| Acacia podalyriaefolia |
|
|---|---|
|
| Acacia polyacantha |
|
|---|---|
|
| Acacia pycantha |
|
|---|---|
|
| Acacia retinodes |
|
|---|---|
|
| Acacia roemeriana |
|
|---|---|
|
| Acacia rigidula |
|
|---|---|
|
| Acacia salicina |
|
|---|---|
|
| Acacia sassa |
|
|---|---|
|
| Acacia schaffneri |
|
|---|---|
|
| Acacia schottii |
|
|---|---|
|
| Acacia senegal |
|
|---|---|
|
| Acacia simplex |
|
|---|---|
|
| Acacia taxensis |
|
|---|---|
|
| Acacia tenuifolia |
|
|---|---|
|
| Acacia tortilis |
|
|---|---|
|
| Acacia sieberiana |
|
|---|---|
|
| Acacia verek |
|
|---|---|
|
| Acacia vestita |
|
|---|---|
|
Anadenanthera
| Anadenanthera colubrina |
|
|---|---|
|
| Anadenanthera macrocarpa |
|
|---|---|
|
| Anadenanthera peregrina |
|
|---|---|
| Tryptamines known to be present in A. peregrina [1] Bufotenine (seeds)Bufotenine N-oxide (seeds)N,N-Dimethyltryptamine N-oxide (seeds)N,N-Dimethyltryptamine (bark)(pods)(seeds)5-Methoxy-N-methyltryptamine (bark)O-Methylbufotenine (bark) (seeds)N-Methyltryptamine (bark) |
Common Reed
| Arundo donax |
|
|---|---|
| Entire Plant contains 5-MeO-DMT (Shulgin, TIHKAL)Flowers contain DMT, 5-MeO-DMT, and 5-MeO-NMT (Shulgin, TIHKAL)Roots contain DMT, 5-MeO-DMT, 5-MeO-NMT, Bufotenine, bufotenidine, dehydrobufotenidine (Shulgin, TIHKAL) |
| Desmanthus illinoensis |
|
|---|---|
| Root contains DMT - 0.200% (Ott)Root Bark contains DMT - 0.340% (Ott) |
| Phalaris arundinacea (Reed Canary Grass) |
|
|---|---|
| Leaves contain DMT, 5-MeO-DMT, and related compounds (Smith 1977) 0.0004-0.121% Total Alkaloids (Dried) Lycaeum (DMT, Life and the Universe) |
| Phalaris tuberosa |
|
|---|---|
| Leaves and seedlings contain DMT, 5-MeO-DMT, and related compounds (Smith 1977)DMT - 0.100% (erowid)5-MeO-DMT - 0.022% (erowid)5-OH-DMT - 0.005% (erowid) |
| Phragmites australis (Common Reed) |
|
|---|---|
| DMT in roots (Ott) |
Delosperma
| Delosperma acuminatum |
|
|---|---|
| DMT, 5-MEO-DMT (Trout's Notes) |
| Delosperma cooperi |
|
|---|---|
| DMT, 5-MEO-DMT (Trout's Notes) |
| Delosperma ecklonis |
|
|---|---|
| DMT (Trout's Notes) |
| Delosperma esterhuyseniae |
|
|---|---|
| DMT (Trout's Notes) |
| Delosperma hallii |
|
|---|---|
| 5-MEO-DMT (Trout's Notes) |
| Delosperma harazianum |
|
|---|---|
| DMT, 5-MEO-DMT (Trout's Notes) |
| Delosperma hirtum |
|
|---|---|
| DMT (Trout's Notes) |
| Delosperma lydenbergense |
|
|---|---|
| DMT, 5-MEO-DMT (Trout's Notes) |
| Delosperma nubigenum |
|
|---|---|
| 5-MEO-DMT (Trout's Notes) |
| Delosperma pageanum |
|
|---|---|
| DMT, 5-MEO-DMT (Trout's Notes) |
| Delosperma pergamentaceum |
|
|---|---|
| Traces of DMT (Trout's Notes) |
| Delosperma tradescantioides |
|
|---|---|
| DMT (Trout's Notes) |
Desmodium
| Desmodium caudatum |
|
|---|---|
|
| Desmodium gangeticum |
|
|---|---|
|
| Desmodium gyrans |
|
|---|---|
|
| Desmodium pulchellum |
|
|---|---|
|
| Desmodium racemosum |
|
|---|---|
|
| Desmodium triflorum |
|
|---|---|
|
| Lespedeza bicolor |
|
|---|---|
var. japonica
|
Petalostylis
| Petalostylis cassioides |
|
|---|---|
| 0.4-0.5% tryptamine, DMT, etc. in leaves and stems (Johns et al 1966) |
Mimosa
General Plant Info
Mimosa hostilis is the former scientific name for Mimosa tenuiflora, and the two names are synonymous [2][3]. The older name is still widely know due to its presence in the literature and as distributers of botanical products still use the older term. M. tenuiflora is an entheogen known as Jurema, Jurema Preta, Black Jurema, and Vinho de Jurema. Dried Mexican Mimosa Hostilis root bark has been recently shown to have a DMT content of about 1%. The stem bark has about 0.03% DMT.
To date no β-carbolines such as harmala alkaloids have been detected in Mimosa tenuiflora decoctions, however the isolation of a new compound called "Yuremamine" from Mimosa tenuiflora as reported in 2005 represents a new class of phyto-indoles [4]. This may explain the reported oral activity of DMT in Jurema without the addition of an MAOI. Imported MHRB typically requires the addition of an MAOI in the preparation of ayahuasca.
Identification
Alkaloid content
- Root Bark contains DMT - 0.31% to 0.57% (Schultes 1977)
- Inner root bark contains up to 2% active alkaloids (Extractions from DMT-Nexus and others)
- 3% of the total alkaloids (or 0.04% of rootbark) is NMT and 2-Methyl-1,2,3,4-Tetrahydro-Beta-Carboline (Analysis of jungle spice, Analysis of red/yellow/white spices
Extraction teks
For extracting DMT , any of the extraction teks described here will work.
Yuremamine is sensitive to heat and pH changes so only cold water (or alcoholic) soak will retrieve it.
Cultivation
Growing: Mimosas aren´t cold proof. For outdoor growing they deserve a sunny place with leachy middle nutrient soil. Throughout the vegetation are copiously watered, in winter the watering is tied down on to the minimum. They are breeding with the seeds, but can be breeded with the cutting also.
Refinery for the Purpose of Extraction
Mimosa Hostilis Root Bark can be acquired in different stages of preparation. Usually it is sold as whole, shredded or pre-powdered root-bark, but one may have access to the whole root—usually when harvested directly.
- The whole root must cleaned and stripped of its inner root-bark while discarding the rest of the root.
- The whole root-bark must generally be torn by hand, cut, or smashed with a blunt object prior to shredding.
- The shredded should be further broken down as much as possible by peeling/cutting/blending to increase surface area for alkaloids to be extracted.
- The pre-powdered can always be used "as-is".
Below details how to break it from whole root
The Root
| Note: | Only the Inner Root Bark is necessary for extraction, the core and outer parts are to be discarded! |
| Root preparation | ||||
|---|---|---|---|---|
|
Pictured below is its original after being harvested from the plant. Notice the middle core is quite distinct from the root-bark, the outer bark is much more brown: |
Cleaning the root
Peeling the Inner Root Bark
| Peeling the Inner Root Bark | |||||
|---|---|---|---|---|---|
|
Once the outermost part has been removed, peel off the Inner Root Bark to separate it from the core. This can easily be accomplished immediately by hand, though the use of a knife may be helpful. Here's the inner core which is to be discarded: |
Result of root preparation
| Result of root preparation | ||
|---|---|---|
|
The peeled inner root-bark now needs to dry. This may be accomplished by simply leaving it in the sun. Here's how it should look: |
Breaking the rootbark up
| Breaking the rootbark up | |
|---|---|
|
The pieces/strips of inner root-bark require further refinery to expose a larger surface area and increase the availability of the alkaloids for extraction. If storage is desired, then the whole pieces are preferable, as the alkaloids are less exposed and thus better protected. First strip the pieces further into thinner layers with the hands, then cut it up with good scissors into smaller squares, then break it down in small amounts and short/medium bursts with a blender or coffee grinder (to prevent breaking of blender/grinder) |
References
- ↑ Southon et al. 1994. Phytochemical Dictionary of the Leguminosae. CRC Press. p. 71.
- ↑ USDA, ARS, National Genetic Resources Program. Germplasm Resources Information Network - (GRIN) [Online Database]. National Germplasm Resources Laboratory, Beltsville, Maryland. URL: http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?24430
- ↑ Lewis, G.P. (1987) Royal Botanic Gardens, Kew 369 pp Legumes of Bahia.
- ↑ Vepsäläinen, Jouko J.; Auriola, Seppo; Tukiainen, Mikko; Ropponen, Nina & Callaway, J.C. (2005). "Isolation and characterization of yuremamine, a new phytoindole". Planta Medica, 71: 1053-1057. URL: http://www.ncbi.nlm.nih.gov/pubmed/16320208
Links
| Mimosa ophthalmocentra (Red Jurema
) |
|
|---|---|
|
1,6% DMT in the inner rootbark [1] |
| Mimosa scabrella |
|
|---|---|
|
| Mimosa verrucosa |
|
|---|---|
|
| Mucuna pruriens |
|
|---|---|
|
| Petalostylis labicheoides |
|
|---|---|
var. casseoides
|
| Diplopterys cabrerana (Chaliponga
) |
|
|---|---|
|
| Horsfieldia superba |
|
|---|---|
|
| Iryanthera ulei |
|
|---|---|
|
| Osteophloem platyspermum |
|
|---|---|
|
Virola
| Virola calophylla |
|
|---|---|
|
| Virola carinata |
|
|---|---|
|
| Virola divergens |
|
|---|---|
|
| Virola elongata |
|
|---|---|
|
| Virola melinonii |
|
|---|---|
|
| Virola multinervia |
|
|---|---|
|
| Virola pavonis |
|
|---|---|
|
| Virola peruviana |
|
|---|---|
|
| Virola rufuta |
|
|---|---|
|
| Virola sebifera |
|
|---|---|
|
| Virola theiodora |
|
|---|---|
|
| Virola venosa |
|
|---|---|
|
| Psychotria carthaginensis |
|
|---|---|
|
| Psychotria viridis (Chacruna
) |
|
|---|---|
|
Sources
- ↑ L. M. Batista; R. N. Almeida; E. V. L. da-Cunha; M. S. da-Silva; J. M. Barbosa-Filho. Isolation and Identification of Putative Hallucinogenic Constituents from the Roots of Mimosa ophthalmocentra. In: Pharmaceutical Biology, Volume 37, Issue 1 January 1999
Extraction Teks
A/B
STB
STB-A/B hybrid
Dry tek
Dosages and consumption methods
History of usage
Scientific publications
Links of interest
What Is DMT?
What Are The Effects?
Depending on the dosage, the effects of DMT can range from a multitude of sensations, from bizarre, beautiful and even 'impossible' visions to literally jaw hanging awe as one is propelled into other dimensions of existence where human language and logic cannot even begin to describe or comprehend.
The following is an excerpt from Psychedelic Monographs and Essays[1] which discusses the different levels of a DMT experience:
Level I: Pre-Hallucinatory experience
This stage is characterized by an interior flowing of energy/consciousness. It may be extremely intense. It may have a positive feeling content.
Level II: Vivid, brilliantly colored, geometric visual hallucinations
Here one is observing a patterned field, basically two dimensional, although it may have a pulsating quality. One may remember having seen this before.
Transitional Phase (Level IIB?): tunnel or breakthrough experience
One may see or fly through a tunnel (a passage to the next level). A veil may part, a membrane may be rent. There is a breakthrough to another world (or perhaps even a series of breakthroughs). Alternatively, it may also happen that the transition from Level II to Level III is abrupt, almost instantaneous, with no experience of transition.
Level III: Three- or higher-dimensional space, possible contact with entities.
This stage is characterized by the experience of being in an "objective" space, that is, a space of at least three dimensions in which objects or entities may be encountered.
Sometimes the entities appear to be intelligent and communicating beings. This stage may be extremely energetic with an experience of everything happening incomprehensibly fast. Alternatively it may be relatively coherent.
Travel is possible at Level III. One may, for example, assume the form and consciousness of a bird, and fly as a bird does (cf. Castaneda, The Teachings of Don Juan pp. 191-196). The limits of of this stage, if any, are unknown. There may be transitions to further stages.
If one would like to read some first hand experiences of DMT journeys, you can read a collection of DMT experience reports at the DMT-Nexus forum.
Is DMT Dangerous?
| Warning: | The risk for health and safety of taking DMT with certain mental and physical illnesses is not undisputed![2] |
"The feeling of doing DMT is as though one had been struck by noetic lightning. The ordinary world is almost instantaneously replaced, not only with a hallucination, but a hallucination whose alien character is its utter alienness. Nothing in this world can prepare one for the impressions that fill your mind when you enter the DMT sensorium.
"The extraordinary brevity of the experience," he continued, "argues that it is incredibly harmless. It virtually disappears from the organism in about ten minutes. The paradox is that DMT is the most powerful yet most harmless of all these things. This is probably because, for reasons which are mysterious to us, DMT occurs in normal brain metabolism (in Serotonin)." -- Terence McKenna
Strangely, even though DMT is one of the most intense psychedelics in existence physically it is one of the safest. Because the human body and mind already contain DMT in trace amounts the brain knows exactly how to utilize and metabolize this entheogen and it leaves the system in a matter of minutes. In fact one could say that since DMT is endogenous to the human nervous system it is the safest psychedelic known, far safer physically than even common alcohol and cigarettes.
Mentally DMT is extremely intense, one of the few dangers that one may encounter is that a person may not be prepared for the intensity of such experience and may have trouble integrating it. It would help a psychonaut immensely if he is well schooled in esoteric and psychedelic states of mind before embarking on any hyper dimensional DMT voyages. Set and Setting also play a big part in facilitating the most positive, insightful and enjoyable experience possible.
How Can One Acquire DMT?
There are a number of ways to acquire this entheogen. The first and most difficult way is to have some substantial chemistry knowledge and experience and actually synthesize pure DMT in a laboratory. This a rather tricky and time consuming process and requires access to some rather obscure and hard to acquire chemicals.
The most common and easiest method to acquire DMT is to extract it from the various plant species that contain the compound. The various plants and extraction techniques can be found on this wiki at the extraction teks.
Reference
Links
Pages in category "DMT"
The following 40 pages are in this category, out of 40 total.
A
BCD
FI |
JLMNPQS |
S cont.T
VW |