Category:DMT
Contents
Brief overview - What is DMT?
NN-Dimethyltryptamine or DMT for short is an short acting psychedelic entheogen which allows a persons consciousness to voyage into the most incredible dimensions, visions, thoughts and experiences imaginable.
It is without doubt one of the most powerful yet mysterious psychedelics in existence but to classify DMT as merely a drug would be doing it a great injustice as DMT is more a trans dimensional key into places and vistas so profound and awe inspiring that it raises many new questions regarding the nature of reality and our place within it.
DMT exists naturally in every human being and also throughout the plant and animal kingdoms. It occurs naturally in many mammals, marine animals, trees, grasses, flowers and shoots.
DMT is closely related to serotonin, the naturally occurring neurotransmitter that psychedelics affect so widely. The pharmacology of DMT is similar to that of other well-known psychedelics. It affects receptor sites for serotonin in much the same way that LSD, psilocybin, and mescaline do. These serotonin receptors are widespread throughout the body and can be found in blood vessels, muscle, glands, and skin.
There are a number of ways to acquire this entheogen. The first and most difficult way is to have some substantial chemistry knowledge and experience and actually synthesize pure DMT in a laboratory. This a rather tricky and time consuming process and requires access to some rather obscure and hard to acquire chemicals.
The most common and easiest method to acquire DMT is to extract it from the various plant species that contain the compound. The various plants and extraction techniques can be found on this wiki at the extraction teks.
Chemical and physical properties
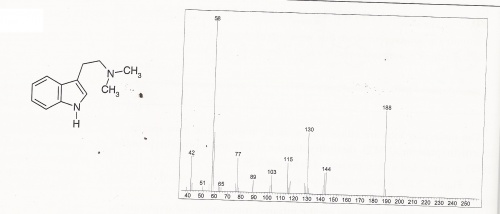
DMT = N,N-Dimethyltryptamine
Freebase DMT
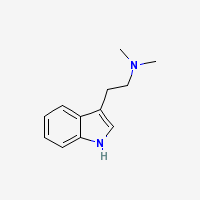 |
- Appearance: White/Transparent crystals
- CAS Registry Number: 61-50-7
- Composition: C12H16N2
- Molecular Weight: 188.26884 g/mol
- Melting point: 44-68°C (Conflicting reports in literature, as mentioned in TIHKAL)
- Boiling point: Theoretically 360-380°C
- XLogP: 2.0
- XLogP3: 2.5 (PubChem)
- pKa: 8.68 (Merck Index)
- Colorimetric reagent results: Here
- Stability/Degradation: Oxidation to DMT N-Oxide (yellow oil) in extended presence of oxygen (specialy in evaporation of dmt-containing solvents with heat and/or fan or generally in prolonged exposure to open air). N-oxide may be reverted back to the parent compound by reduction, as described in the N-Oxide to Freebase Wiki.
- Solubility:
Very Soluble in Xylene, Toluene, Limonene, acetone, Isopropyl Alcohol (IPA), methanol, ethanol, Dichloromethane (DCM), chloroform, ether, Butanone (also known as methyl ethyl ketone (MEK)) and butanol.
Soluble in naphtha, hexane, heptane but almost insoluble in these solvents at freezing temperatures
Almost insoluble in water.
DMT N-Oxide
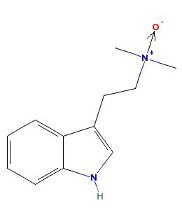 |
- Appearance: Yellow oil
- XLogP3: 2
- Colorimetric reagent results: Here
- Solubility:
Soluble in Xylene, Toluene, Limonene
Slightly soluble in basic water
Insoluble in naphtha
DMT Fumarate
- Molecular Weight: 492.608 g/mol
- Solubility:
Very soluble in water
Soluble in methanol (~10mg/ml)
Soluble in boiling IPA, Practically insoluble in room temp IPA (~1mg/ml), Insoluble in freeze-cold IPA.
Slightly soluble in ethanol (~5mg/ml)
Insoluble in cold acetone
Insoluble in FASI (Fumaric Acid Saturated IPA)
Insoluble in FASA (Fumaric Acid Saturated Acetone)
Effects
Depending on the dosage, the effects of DMT can range from a multitude of sensations, from bizarre, beautiful and even 'impossible' visions to literally jaw hanging awe as one is propelled into other dimensions of existence where human language and logic cannot even begin to describe or comprehend.
There have been a few attempts to define different levels and types of experience. Psychedelic Monographs and Essays[1] discusses different levels of a DMT experience.
The Hyperspace Lexicon project is an attempt to create a new vocabulary to try to describe the DMT realm
Pharmacology, toxicity and general safety
For info on DMT safety, please reffer to Health and Safety section
Plants containing DMT
The following is a list of plants known to contain tryptamines. In some, the DMT content may be very small or it may be present together with other potentially unwanted alkaloids. Please research well before extracting from some plant, and be sure you have your desired alkaloids only when bioassaying from a new plant.
Acacia
- DMT in the leaf (Trout's Notes)
- 0.2% tryptamine in bark, leaves, some in flowers, phenylethylamine in flowers (Hegnauer 1994)
- DMT in plant (Lyceaum)
- Bark of A. maidenii contains 0.6% of N-methyltryptamine and DMT in the proportions approx. 2:3 (Fitzgerald & Sioumis 1965)
- DMT in the bark and leaf, less than 0.02% total alkaloids (Hegnauer 1994)
- DMT in the leaf (Trout's Notes)
- DMT in the leaf (Trout's Notes)
- 0.4 to 0.5 % DMT/NMT in the dried bark (Csiro 1990)
- 0.15-0.6% DMT,NMT(2:1)plus trace betacarboline in bark, 0.06-0.2% leaves (Southern Cross University comissioned test 2001)
- 5-MeoDMT & bufotenine in some loctations (E., Entheogen Review 1995-6; Trout's Notes 2005-10) Is not fast growing in the wild and is under threat of serious overharvesting. Is NOT considered a weed as previously stated here, and will become rarer if wild seed populations exploited further.(Nen, original bioassay subject)
- Less than 0.1% DMT in leaf (Ott)
- 0.3% DMT in leaf, NMT (Trout's Notes)
- Tryptamine in the leaf (Trout's Notes)
- 0.5% to 2% DMT in fresh bark, phenethylamine trace amounts (Hegnauer 1994)
- DMT in leaf (Trout's Notes)
- DMT and MMT (www.factorey.ch/Eins.htm)
- Less than 0.02% total alkaloids found (Hegnauer 1994)
- DMT, NMT, tryptamine, amphetamines, mescaline, nicotine and others (Phytochem. 199
- DMT, in the leaf
- DMT and NMT, in the leaf, stem and trunk bark, 0.81% DMT in bark, MMT
- DMT, NMT, and other tryptamines
- DMT in the leaf (Trout's Notes)
Anadenanthera
- Seed pods contain dimethyltryptamine and the seeds bufotenin, bufotenin oxide, and oxide of dimethyltryptamine (GRANIER-DOYEUX 1965)
Common Reed
- Entire Plant contains 5-MeO-DMT (Shulgin, TIHKAL)Flowers contain DMT, 5-MeO-DMT, and 5-MeO-NMT (Shulgin, TIHKAL)Roots contain DMT, 5-MeO-DMT, 5-MeO-NMT, Bufotenine, bufotenidine, dehydrobufotenidine (Shulgin, TIHKAL)
- Plants analyzed in india were found with alkaloids, others from USA where not found to contain DMT based alkaloids (Trout notes)
- Root contains DMT - 0.200% (Ott)Root Bark contains DMT - 0.340% (Ott)
- Leaves and seedlings contain DMT, 5-MeO-DMT, and related compounds (Smith 1977)DMT - 0.100% (erowid)5-MeO-DMT - 0.022% (erowid)5-OH-DMT - 0.005% (erowid)
Delosperma
- DMT, 5-MEO-DMT (Trout's Notes)
- DMT, 5-MEO-DMT (Trout's Notes)
- DMT (Trout's Notes)
- DMT (Trout's Notes)
- DMT, 5-MEO-DMT (Trout's Notes)
- DMT (Trout's Notes)
- DMT, 5-MEO-DMT (Trout's Notes)
- DMT, 5-MEO-DMT (Trout's Notes)
- Traces of DMT (Trout's Notes)
- DMT (Trout's Notes)]]
Desmodium
- Roots: 0.087% DMT, Bufotenine-N-oxide 0.03% (Trout's Notes)
- DMT, 5-MEO-DMT, whole plant, roots, stems, leaves (Ott)
- DMT, 5-MEO-DMT, leaves, roots (Ott)
- DMT, 5-MEO-DMT, whole plant, roots, stems, leaves, flowers (Ott)
- 5-MEO-DMT (Ott)
- DMT-N-oxide, roots (Ott)
Lespedeza bicolorvar. japonica
- DMT, 5-MEO-DMT in leaves and root bark (Ott)
Petalostylis
- 0.4-0.5% tryptamine, DMT, etc. in leaves and stems (Johns et al 1966)
Mimosa
- 1.6% DMT in rootbark
- DMT in bark (Ott)
- DMT (Schultes 1969)
- Leaves, seeds, stems and roots contain L-Dopa, Serotonin, 5-HTP, and Nicotine, as well as N,N-DMT, Bufotenine, and 5-MeO-DMT (Erowid)
Petalostylis labicheoidesvar. casseoides
- DMT in leaves and stems (Ott)
- DMT
- DMT, 5-MEO-DMT in bark (Ott)
Virola
- Leaves 0.149% DMT (Ott)
- DMT in leaves (Ott)
- DMT in leaves (Ott)
- DMT, 5-MEO-DMT in bark and leaves (Ott)
- DMT in bark (Ott)
- DMT, 5-MEO-DMT in bark and leaves (Ott)
- DMT in leaves (Ott)
- DMT, 5-MEO-DMT in bark (Ott)
- Alkaloids in bark and root, 95% of which is 5-MeO-DMT (Shulgin, TIHKAL)
- DMT in bark (Ott)
- DMT, 5-MEO-DMT in bark, roots, leaves and flowers (Ott)
- DMT, 5-MEO-DMT in roots and leaves (Ott)
- 0.2% average DMT in dried leaves (Ott)
Extraction Teks
A/B
STB
STB-A/B hybrid
Dry tek
Dosages and consumption methods
History of usage
Analysis of DMT
Colorimetric reagents
LC / GC-MS
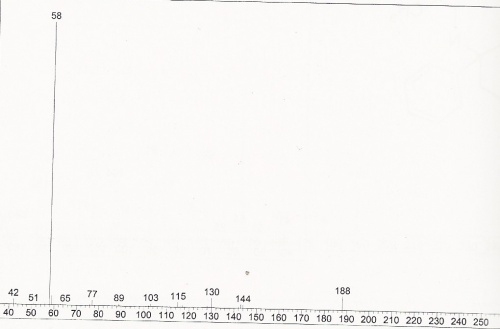
DMT Mass Spectra
DMT Mass Spectra (expanded)
Scientific publications
Links of interest
Cite error: <ref> tags exist, but no <references/> tag was found
Pages in category "DMT"
The following 40 pages are in this category, out of 40 total.