The Nexian DMT Handbook
| Note: | This page is a work in progress -- its content throughout is not yet complete. |
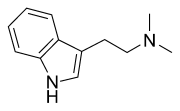
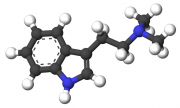
The production and use of DMT (N,N-Dimethyltryptamine), otherwise known as "Spice", is a practice that resonates strongly with the complementary qualities of ancient shamanic and alchemical spiritual practice as well as contemporary DIY (Do It Yourself) ethic. The production of spice is a discipline unlike that of most other commonly manufactured drugs, as it is not as well suited for bulk-production nor production for the purpose of sale as most well-known and intensively manufactured substances. As such, its use is generally inseparable from its production in practice and in spirit.
The production of DMT most commonly entails its extraction from botanical sources and only very rarely entails its synthesis. In this way, its production still strongly resembles its more ancient preparations by manner of brewing, a simple form of aqueous extraction still commonly performed to this day. This is the simplest and most readily administered form of extract if used as a component of a harmaloid-based preparation or -huasca brew.
Please take the time to seek further elaboration at the following resources:
Contents
- 1 Source Selection
- 2 General Plant Info
- 3 Identification
- 4 Alkaloid content
- 5 Extraction teks
- 6 Cultivation
- 7 Refinery for the Purpose of Extraction
- 8 References
- 9 Links
- 10 Extraction
- 11 Crystallization
- 12 The FASA Method
- 13 Purification
- 14 Administration
- 15 Appendices
Source Selection
DMT, its analogues, and other related alkaloids can be found in a wide variety of lifeforms, varying from trace amounts to considerable amounts. It is impossible, therefore, to include all of the sources from which DMT can be extracted, so the following discussion will focus primarily on the most commonly used and significant botanical sources.
Botanical Considerations
Several species of plants contain a variety of constituents apart from DMT. This consideration is of the utmost importance when selecting the source plant from which an extraction is to be performed, as it may become the determining factor in the material requirements of the extraction process. Some plants may even contain toxic alkaloids, so thorough research must be conducted prior to selection, extraction, and administration.
Common Botanical Sources
General Plant Info
Mimosa hostilis is the former scientific name for Mimosa tenuiflora, and the two names are synonymous [1][2]. The older name is still widely know due to its presence in the literature and as distributers of botanical products still use the older term. M. tenuiflora is an entheogen known as Jurema, Jurema Preta, Black Jurema, and Vinho de Jurema. Dried Mexican Mimosa Hostilis root bark has been recently shown to have a DMT content of about 1%. The stem bark has about 0.03% DMT.
To date no β-carbolines such as harmala alkaloids have been detected in Mimosa tenuiflora decoctions, however the isolation of a new compound called "Yuremamine" from Mimosa tenuiflora as reported in 2005 represents a new class of phyto-indoles [3]. This may explain the reported oral activity of DMT in Jurema without the addition of an MAOI. Imported MHRB typically requires the addition of an MAOI in the preparation of ayahuasca.
Identification
Alkaloid content
- Root Bark contains DMT - 0.31% to 0.57% (Schultes 1977)
- Inner root bark contains up to 2% active alkaloids (Extractions from DMT-Nexus and others)
- 3% of the total alkaloids (or 0.04% of rootbark) is NMT and 2-Methyl-1,2,3,4-Tetrahydro-Beta-Carboline (Analysis of jungle spice, Analysis of red/yellow/white spices
Extraction teks
For extracting DMT , any of the extraction teks described here will work.
Yuremamine is sensitive to heat and pH changes so only cold water (or alcoholic) soak will retrieve it.
Cultivation
Growing: Mimosas aren´t cold proof. For outdoor growing they deserve a sunny place with leachy middle nutrient soil. Throughout the vegetation are copiously watered, in winter the watering is tied down on to the minimum. They are breeding with the seeds, but can be breeded with the cutting also.
Refinery for the Purpose of Extraction
Mimosa Hostilis Root Bark can be acquired in different stages of preparation. Usually it is sold as whole, shredded or pre-powdered root-bark, but one may have access to the whole root—usually when harvested directly.
- The whole root must cleaned and stripped of its inner root-bark while discarding the rest of the root.
- The whole root-bark must generally be torn by hand, cut, or smashed with a blunt object prior to shredding.
- The shredded should be further broken down as much as possible by peeling/cutting/blending to increase surface area for alkaloids to be extracted.
- The pre-powdered can always be used "as-is".
Below details how to break it from whole root
The Root
| Note: | Only the Inner Root Bark is necessary for extraction, the core and outer parts are to be discarded! |
| Root preparation | ||||
|---|---|---|---|---|
|
Pictured below is its original after being harvested from the plant. Notice the middle core is quite distinct from the root-bark, the outer bark is much more brown: |
Cleaning the root
Peeling the Inner Root Bark
| Peeling the Inner Root Bark | |||||
|---|---|---|---|---|---|
|
Once the outermost part has been removed, peel off the Inner Root Bark to separate it from the core. This can easily be accomplished immediately by hand, though the use of a knife may be helpful. Here's the inner core which is to be discarded: |
Result of root preparation
| Result of root preparation | ||
|---|---|---|
|
The peeled inner root-bark now needs to dry. This may be accomplished by simply leaving it in the sun. Here's how it should look: |
Breaking the rootbark up
| Breaking the rootbark up | |
|---|---|
|
The pieces/strips of inner root-bark require further refinery to expose a larger surface area and increase the availability of the alkaloids for extraction. If storage is desired, then the whole pieces are preferable, as the alkaloids are less exposed and thus better protected. First strip the pieces further into thinner layers with the hands, then cut it up with good scissors into smaller squares, then break it down in small amounts and short/medium bursts with a blender or coffee grinder (to prevent breaking of blender/grinder) |
References
- ↑ USDA, ARS, National Genetic Resources Program. Germplasm Resources Information Network - (GRIN) [Online Database]. National Germplasm Resources Laboratory, Beltsville, Maryland. URL: http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?24430
- ↑ Lewis, G.P. (1987) Royal Botanic Gardens, Kew 369 pp Legumes of Bahia.
- ↑ Vepsäläinen, Jouko J.; Auriola, Seppo; Tukiainen, Mikko; Ropponen, Nina & Callaway, J.C. (2005). "Isolation and characterization of yuremamine, a new phytoindole". Planta Medica, 71: 1053-1057. URL: http://www.ncbi.nlm.nih.gov/pubmed/16320208
Links
| Diplopterys cabrerana (Chaliponga
) |
|
|---|---|
|
| Psychotria viridis (Chacruna
) |
|
|---|---|
|
See also:
Methods of Refinery
Before extracting alkaloids from the variety of plants named above, one generally needs to clean and prepare the plant source, to include washing, pulverization, or any other necessary pre-treatment. Oftentimes, plants obtained through vendors have been prepared to some extent, and only require some degree of pulverization unless obtained in pre-powdered form. Pulverization is generally achieved by use of a household coffee grinder or a blender, though some materials must be prepared for pulverization by hand or any other method to avoid damaging the grinder. The process can be quite messy and painstaking, depending on the material an methods used.
Defatting Concerns
Depending on the extraction methods used, foliage will require a defatting phase in order to better facilitate a proper extraction. This generally reguires that the material undergo extraction by acidic water prior to defatting. The product will be rendered water soluble and will remain in an aqueous phase as the solution is washed with an NPS to remove fats and oils.
Vendor Considerations
Though none of the plants containing DMT are illegal, the alkaloids contained within generally are. Vendors generally must take it upon themselves not to advertise the illicit contents of their products, otherwise risking putting themselves and their customers in jeopardy. Ordering these botanicals from across borders into countries where their contents are considered illegal is ill-advised but not necessarily risky, especially not if intended for legitimate use. Always be sure to research the reputation of a vendor prior to purchase, as the potentially taboo nature of their products may inspire unethical business practice, such as unreliable delivery or poor product quality; though such cases are not necessarily specifically limited specifically to these sorts of vendors, of course.
Considerations Regarding Cultivation
Again, as these plants are usually no illegal, neither is their cultivation. Often these plants are only native to very specific regions, thus requiring very specific growing conditions, but many can be successfully cultivated indoors or seasonally outside of their native regions. Cultivation for the expressed or deduced purpose of refinery, use or sale of an illicit substance can result in harsh legal consequences, however.
Extraction
Extraction generally refers to the process of isolating a product from a source. The basic idea is to utilize the unique properties of the product—whether reactive, electromagnetic, or otherwise structural—to draw it out of the source and into a target solvent. To accomplish this the product must either be naturally soluble in the solvent or must undergo reaction to increase its solubility. The difference between a high and low yield is firstly determined by how much more soluble the product is in the target solvent, than in its source material or solution, and secondly by how thoroughly the target solvent is mechanically brought into contact with the target solvent; this is generally determined by a combination of the droplet size and dispersion of a solvent and/or the surface area exposure and structural consistency of the source material.
See also:
Straight-To-Base Extraction
Straight-To-Base or STB techniques are generally considered the simplest of extraction techniques and, as such, are the most commonly used. The process involves the use of a strong base reagent in solution to break down source material and convert the contained product from its natural salt form to freebase, which will in turn be more soluble in an NPS than in the basic aqueous solution.
Considerations:
- The use of "Straight To Base" techniques requires little experience or technical know-how for beginners to approach extraction techniques. STB is best-suited for quick, non-labor-intensive, crude bulk extractions. It requires no straining or cooking but requires time for soaking and separation. STB tends to yield a greater array of botanical impurities due to its lack of straining and defatting. These techniques do however enable a more thorough exhaustion of product from the material. This technique is ideal for shredded material that requires little or no defatting.
Overview of Materials and Methods
| Materials Required | |
|---|---|
Source Material:
|
|
Solvents:
|
|
Reagents/Desiccants:
|
|
Equipment:
|
Material Considerations:
- Lye is extremely toxic and hazardous, though most find it easy to handle safely.
- STB methods generally demand a hefty volume of solvents for sufficient performance.
- Naphtha and heptane are generally found to pull a more pure product, and though their poor ability to dissolve DMT demands some amount of heating to pull a considerable amount, it also allows for product to be isolated by freeze-precipitation.
- Toluene and Xylene are generally found to dissolve DMT more easily but also dissolve a wider range of alkaloids and other substances from the source material, and though they do not facilitate freeze-precipitation, they do facilitate the use of FASA.
- DCM is capable of pulling an even fuller range of alkaloids from the source material, as unlike other NPS's, it sinks in water.
- Diethyl ether as a binary solvent in combination with heptane also pulls full-range alkaloids. Using this combination the ether can be distilled off rather rapidly, leaving behind an alkaloid-saturated solution of heptane ready for immediate freeze precipitation. Note! Diethyl ether is extremely flammable. Exercise extreme caution!
- Limonene has become the solvent of choice among many--largely due to being nontoxic--but is considerably more expensive and harder to find than other less savory NPS's, and though it does not facilitate the use of FASA or freeze-precipitation, it can facilitate salting methods like FASW and FASIPA.
- Most commonly used solvents do not facilitate expedient or clean evaporation, due to either impurities from the solvent itself or pulled from the source material, though heptane and naphtha tend to be most effectively used in this way.
Methods:
- STB extraction involves a soak of pulverized source material in a basic solution, followed by the stirring in of an NPS. The NPS is separated from the aqueous solution by siphoning, pipetting, or use of a separatory device.
Material Preparation
For standard STB, the source material must generally be at least shredded, though preferably powdered. It has been found beneficial to pre-treat the pulverized material with an acid soak, with or without heat, prior to immersing in a basic solution.
Extraction Procedure
- Prepare a basic solution between pH 12-14 by adding base to the appropriate amount of water for the amount of source material to be used.
- Immerse the pulverized source material in the basic solution and allot time for material breakdown and freebase conversion.
- Stir or shake in the target solvent enough to ensure adequate dissolution of product.
- Allow the solvent to separate from the aqueous solution in two distinct layers and separate using appropriate separatory methods.
- Collect solvent and proceed with appropriate desired crystallization methods.
Further Elaboration and Technical Support
Acid/Base Extraction
Acid/Base or AB extractions use a weak acid(citric, acetic, and OTHERS have been used with success) to extract the DMT from the plant material into DMT freebase.
Considerations:
- The use of Acid/Base techniques implies the use of "acid-cooking" the source material, straining it, and basifying the resulting strained solution. The use of an initial acid extraction facilitates the implementation of a defatting phase and generally yields a product more devoid of botanical impurities. This technique is ideal for any material that requires defatting, though defatting may not be necessary, depending on the intended method of crystallization.
Overview of Materials and Methods
| Materials Required |
|---|
Methods:
Material Preparation
Either purchase shredded bark or Break 1-Pound of Mimosa Hostilis rootbark into 2" pieces and grind it all up in a glass-topped blender, a little at a time.
Extraction Procedure
(taken from tek) Premix in an empty 1-Gallon plastic jug: 1-Pint White Vinegar & 3-1/2 Quarts Water. Put the ground up Mimosa in a 3-Liter crockpot, then fill it with the water-vinegar solution.
Stir well and turn it on "high". After 2 hours, remove the crockpot ceramic liner, hold the lid on slightly offset, and pour off most of the liquid into a 1-gallon wide-mouthed glass or stainless container.
Add the remaining water-vinegar solution to the crockpot again. Stir well and turn it on "high". After 2 hours, remove the crockpot ceramic liner, hold the lid on slightly offset, and pour off all of the liquid into the same container again. Discard the rootbark fiber and save the two combined extractions in the 1-gallon container.
Allow the vegetable particles in the extraction in the 1-gallon container to settle to the bottom overnight. Then pour off the liquid into an empty 1-Gallon GLASS wine jug, being careful not to pour off any of the vegetable sludge at the bottom. Discard the sludge and keep the contents of the wine jug.
Premix in advance a solution of: 4 Tablespoons (50grams) of Sodium Hydroxide ("Red Devil" lye) with 1-Pint of HOT Water. Stir well. Slowly add this solution to the wine jug, then cap the jug. Gently tilt the wine jug back and forth for 1 full minute to mix the contents.
from here use your solvent of choice
Further Elaboration and Technical Support
Dry Technique Extraction
| Note: | This technique is still experimental and may result in variable success if used with MHRB. |
Dry techniques (drytek) evolved from and are ideally intended for the implementation of the FASA method of crystallization and serve as the only techniques able to implement acetone as an extraction solvent. Acetone is generally favored for its ability to extract a notably broad range of active products.
Considerations:
- The use of dry techniques requires fewer and less toxic materials than the techniques that employ aqueous phases and separatory methods. The materials required are generally of a more household nature. They are most effectively applied to powdered botanical material. Acetone is, however, completely water miscible, so proper drying procedures are of the utmost importance. This technique may or may not require the defatting of botanical materials, depending on the intended method of crystallization. Dry techniques are the youngest of the current extraction techniques though apparently sound in theory and in practice.
Overview of Materials and Methods
| Materials Required | |
|---|---|
Source Material:
|
|
Solvents:
|
|
Reagents/Desiccants:
|
Material Considerations:
- Acetone can be purchased at hardware stores but should be confirmed as pure acetone prior to purchase. Note that almost all acetone can contain up to 5% water contamination, depending on time and shelving conditions.
- In order to be utilized for extraction, sodium bicarbonate must undergo conversion to sodium carbonate.
- Lime is often found difficult to decant acetone off of and also difficult to filter out of acetone, whereas sodium carbonate is generally found more agreeable for both.
- Distilled water is preferable, as tap water almost always contains impurities that can potentially tamper with resulting yields.
- With few exceptions, the source material should be completely pulverized to a powder consistency before use, as this technique's choice of reagents are not quite capable of penetrating cell structure.
Methods:
- Extractions by dry techniques are characterized by the lack of a traditional aqueous phase in the extraction process, and instead, opting for basification within a paste which is followed by chemically drying the paste with desiccant. The process does not make use of separatory methods, and instead is characterized by it's use of dry-washing, decanting and non-intensive filtering methods. Certain materials must be rendered anhydrous prior to use.
Material Preparation
| Rendering Anhydrous Magnesium Sulfate | ||||
|---|---|---|---|---|
|
| Rendering Anhydrous Acetone | |
|---|---|
|
| THP Approach to Rendering Anhydrous Acetone | |
|---|---|
|
| Conversion of Sodium Bicarbonate into Sodium Carbonate | ||||
|---|---|---|---|---|
|
Extraction Procedure
- Mix the intended base with the powdered source material at a ratio between 1:2 and 1:1.
- The product remains in its natural salt form which is generally considered to be quite free from the botanical cell structure in powdered material.
- Add only enough water to thoroughly moisten the mixture to the consistency of a paste while stirring to ensure the consistency of the mixture.
- Although this is not generally considered a traditional aqueous phase in that it is not a solution, it is an aqueous phase in that it is excessively hydrated and sufficiently aqueous to facilitate reaction.
- Allow adequate time to soak in order for reaction to occur.
- The acid component of salt-form product undergoes reaction with the base, effectively neutralizing the acid and freeing the product in its pure alkaloid form, or freebase.
- Stir in anhydrous magnesium sulfate until thoroughly dry.
- The magnesium sulfate acts as a desiccant, and that this is performed in order to prevent water contamination of the acetone.
- Add an excess of anhydrous acetone and stir thoroughly, allotting adequate time and stirring for thorough dissolution of the product into the acetone.
- The more contact allotted between the product and the acetone, the greater the saturation.
- Decant and/or filter acetone and collect, being careful not to allow any particulates into the collection vessel.
- The bases used should not harm the quality of the product, but may interfere with the accuracy of weight.
- Repeat steps 5-6 with fresh acetone until material is exhausted to satisfaction, and proceed to the appropriate desired crystallization method.
- Three washes is generally considered sufficient.
Further Elaboration and Technical Support
Limtek Extraction
Though all of the other extraction techniques may be employed in nontoxic or at least less toxic manners, few are perfectly suited for completely nontoxic implementation with food-grade household chemicals. Limtek extraction is named as such because it employs the use of d-Limonene and pickling lime, and is distinguished by the unique way in which lime must be used for effective results--similarly to drytek--as well as the hygrophobic properties of limonene. Unlike most bases used in extraction, lime has very low solubility in water, and so even though it does qualify as a strong base, it does not behave as such in solution; it must be mixed into a paste with the source material and a minimal amount of water in order to behave as a strong base. One of the drawbacks of drytek extraction is that upon removal of moisture from the mixture of material and reagent, the reaction will essentially terminate, greatly limiting the effectiveness of the extraction. Limonene, however, is an NPS and so is hygrophobic, meaning that the source material can remain moist.
Considerations:
- Limtek extraction is nontoxic and food-grade throughout, using no toxic or otherwise hazardous materials in the process. This technique uses the absolute minimum of material and volume possible for extraction, and due to it's lack of a proper aqueous phase in the extraction process, requires no separatory methods prior to salting and facilitates solid disposal in lieu of dumping large volumes of potentially toxic solution. This process bears the least resemblance to the production of other less savory substances, reducing legal risks to the operator, to include chemical odors.
Overview of Materials and Methods
| Materials Required | |
|---|---|
Source Material:
|
|
Solvents:
|
|
Reagents/Desiccants:
|
Material Considerations:
- In order for the process to remain nontoxic, an aqueous food-grade acid should be used to salt out of the product.
- FASW (Fumaric Acid Saturated Water) can be evaporated to achieve a crystalline DMT fumarate.
- vinegar can be used to achieve DMT acetate but will not crystallize.
- Limonene can be replaced with other NPS's, but the process will cease to be nontoxic.
Methods:
- Extraction by limtek is characterized by the lack of a proper aqueous phase but also the lack of necessity for drying procedures, and essentially involves a pasty or doughy material being washed in the target solvent to retrieve product.
Extraction Procedure
- Mix the powdered material with lime between 1:2 and 1:1 and mix thoroughly.
- no reaction occurs at this point but thorough mixing will help to ensure an even reaction for the following steps.
- Thoroughly moisten the mixture while stirring, ensuring that no dry spots remain and the mixture exhibits a doughy or pasty consistency.
- the added water facilitates reaction and a basic environment which may aid in breaking down the plant material.
- the acid component of the natural salt-form of the product within the plant material will be neutralized, yielding a freebase product.
- Ensure a thoroughly homogeneous mixture and allot enough time and stirring for a complete reaction.
- this is the most important part for a successful yield, and due to the lack of a proper aqueous phase, the reaction requires a stronger degree of manual facilitation.
- Wash the mixture with NPS by stirring and decanting to collect the NPS, and proceed to the appropriate desired crystallization method.
- the more contact brought between the material and the target solvent, the more successful the extraction will be.
Further Elaboration and Technical Support
- Directory of Current Limteks
- A Poll to Gauge Members' Success w/ Lime
- Pickling lime instead of lye?
- Calcium Hydroxide instead of lye?
Crystallization
Crystallization is the process by which a product is isolated from a solvent. This is accomplished by either allowing the solvent to completely evaporate or by causing a precipitation to occur within the solvent, which can then be isolated from the solvent by several methods and then dried of any residual solvent.
Evaporation
In extraction, evaporation is the process by which a solvent disperses from its liquid form into the air as a vapor and a gas. When this occurs, the less volatile constituents of the solvent solution are left behind, and as such, it is a common method of isolating solutes from solvent.
Considerations:
- Many common solvents contain impurities which may not be quite as volatile as the pure solvent and may leave these impurities behind as a residue.
- Solvents often emit fumes and odors which may be hazardous to health, flammable, or may alarm those within proximity of the odor.
- Some solvents may require an excessive length of time to evaporate.
- Some solvents may absorb ambient moisture, resulting in a less expedient evaporation.
- Excessive air flow may cause the oxidization of the product.
- Solvent may become Trapped within the crystal structure of the product, resulting in a less solid and less pure product.
Freeze Precipitation
Freeze precipitation is the process by which product is isolated from a solvent through a decrease in solubility achieved by lowering the temperature of the solvent. This process generally relies on the solvent being completely saturated or super-saturated with product. Freeze precipitation is generally the fastest method by which product can be isolated immediately following extraction, but it relies on the use of only very specific solvents. This method is preferably used in conjunction with A/B and STB techniques though can be used alongside variations of drier techniques.
Salting
Salting is the process by which freebase DMT is reacted with an acid to create a salt form which is generally water-soluble. The natural form of DMT in botanical sources tends to be a salt-form, thus facilitating the simple aqueous extraction used to prepare DMT-containing brews. It is quite common to perform aqueous acid extractions from the material, however—whether for the purposes of a brew or for A/B extraction. The salt-form itself rarely lends itself to proper crystallization and usually can only be isolated as an oil unless very specific methods and materials are employed.
The Use of Fumaric Acid
Few salt-forms of DMT facilitate crystallization, but DMT fumarate currently stands alone as a solid crystalline salt-form of DMT.
The FASA Method
| Materials Required | |
|---|---|
Source Material:
|
|
Solvents:
|
|
Reagents/Desiccants:
|
The FASA, or fumaric acid saturated acetone, method is a method employed to render DMT Fumarate.
Considerations:
- DMT Fumarate is reportedly quite stable and resistant to oxidization or other forms of degradation. It is notably resistant to heat, and as such is able to withstand low-temperature oven-drying. Certain other related compounds, such as jungle-spice and bufotenine are also able to crystallize as a fumarate. Defatting is not required prior to employing FASA methods, as oils and most other impurities should not interfere with this method's procedure or the yield
- Because DMT Fumarate is water-soluble, it is also well-suited for oral administration in conjunction with harmaloids, either mixed into a beverage or encapsulated.
Methods:
- The FASA method employs the firstly, the solubility of fumaric acid in acetone, and secondly, solubility of freebase DMT in acetone, and thirdly, the insolubility of DMT Fumarate in acetone or the non-polar solvents commonly utilized for extraction. The solubility of both DMT and fumaric acid in acetone facilitates their reaction to produce a crystalline DMT salt which is completely insoluble in acetone or non-polar solvents.
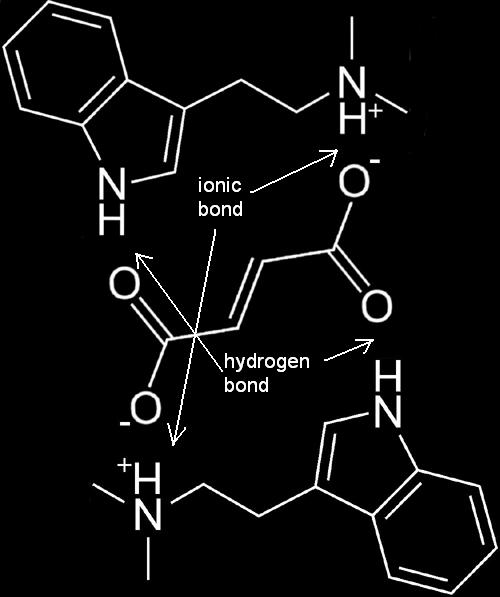
FASA Molecule
One fumaric acid molecule attaches to two DMT freebase molecules.
Procedure
| Rendering Crystalline DMT Fumarate | |
|---|---|
|
Links
- The FASA Method: A Summary - DMT Fumarate and Beyond
- DMT Fumarate to DMT Freebase
- FASA Alteration of Final Purification
- Fumarates to Freebase Conversion TEK
- Spice Extraction-The FASA Approach
The FASW Method
this is a procedure to salt a 100-300ml pull of limonene the measurments are more of a rough guideline it doesnt have to be exact play around with what works best for you... Get a small jar/beaker/cylindrical container and put around 300ml of WARM water in it. Add ~2g of fumaric acid to it. Stir this like crazy. There may be a few fumaric acid chunks in there but can leave them and fill with more warm water for later pulls.
Stir 50ml FASW into the d-limonene for at least 10 minutes, HARD. Then suck out/drain off the dmt fumarate saturated water, which will be the bottom liquid layer. SWIM usually cleans out the third small jar and puts this saturated water in it before putting the water on the evaporating plate. This is so that he can filter the water before it hits the evaporating plate. This keeps any traces of d-limonene out of the soon to be evaporated dmt water. Repeat this step two or three more times with 50ml fresh FASW each time per pull of limo
Evaporate the saturated dmt water in the dehydrator or on a very very low oven setting, no more than 175F. This will result in the solid DMT fumarate. This stuff is HARD. It may be difficult to scrape up, but just use some muscle and it will come up. from here you can freebase or just eat the fumarate like i do ;]
Note: the solubility of fumaric acid in 100ml water is 0.63 g @ 25°C, 1.07 g @ 40°C, 2.4 g @ 60°C, 9.8 g @ 100°C. (The Merth Index, 8th ed.)
The FASIPA Method
Take your small jar and put 5g fumaric acid in it and then fill the jar with your 99% IPA. Then mix this together until the IPA is completely saturated. This creates Fumaric Acid Saturated IPA(FASI). Most likely you will have excess fumaric acid on the bottom but that is fine. Now SLOWLY pour your FASI into your jar of dmt saturated d-limonene. The slower the better. For best results add 10ml FASI every 5 minutes until you dont see any more cloud forming in the d-limonene. This will crash out your dmt from the d-limonene. Allow this to sit for at least 24 hours after you stop putting in FASI so that the DMT Fumarate can fully crash out. After 24 hours add another 1ml of FASI to make sure all dmt has precipitated out. After you collect the DMT fumarate from the jar, put the d-limonene back into the jar and let it sit for a few days. More DMT fumarate will precipitate over time. Now pour off the d-limonene from your now beautiful crystals. Turn the jar upside down and put it on a paper towel to absorb any extra D-Limonene that drips out. Allow this to sit out until it dries a bit more. You can use a bit of heat and a fan to speed this process up. Now scrape up your JIMJAM fumarate. It will smell mildly of oranges. DONE
Discussion of Other Salt-Forms
Further Elaboration and Technical Support
Purification
The purification of DMT product has several purposes and is accomplished by several different methods, but all of them essentially involve the washing of product in some way or another. Purification either involves the isolation of product from unwanted impurities from the plant source or from the process of extraction, or it involves the isolation of product from active impurities which may or may not be collected after isolation.
Recrystallization
The general purpose of recrystallization is to crystallize the product in a fresh solvent after it has already been isolated from a solvent containing a considerable amount of impurities. This is meant to lessen the interference of impurities on the process of crystallization. Often this process results in more well-formed crystals with less discoloration. The advantage of this method of purification is that the solvent choice for recrystalliztion may be different and more suitable than that chosen for extraction.
Washing
The purpose of washing is to disperse impurities off of the product or out of a solution containing the product and into an intermediate solvent.
Alkaline Solution Washing of Inactive Impurities
Most of the impurities that plague yields tend to be quite soluble in both alkaline aqueous solutions and non-polar solvents. To remove these impurities, an imbalance in equilibrium must be created between these two types of solutions, causing the impurities to disperse into a disposable solution from the solution containing the product.
Solvent Washing and Isolation of Active Impurities
Active impurities require a slightly different method of isolation for purification and generally rely strictly on differences of their solubility or insolubility in specific solvents. Often, reactions are required in order to create these differences, as the products tend to exhibit very similar properties.
Freebase Conversion from Salt
The methods used for converting crystalline salt-form DMT into freebase are not dissimilar from those used in extraction. The only significant difference between the processes is that the conversion involves far fewer impurities and less material than the extraction. Because of of this and the fact that it involves the isolation of the product from an acid, the conversion acts as a sort of purification method.
Drytek Conversion
This conversion is preferred for it's lack of need for separatory methods and for it's notably dry quality which facilitates the use of acetone. This method of conversion evolved out of the FASA method and is characteristically identical to Drytek extraction.
| Drytek Freebase Conversion of DMT | |||||
|---|---|---|---|---|---|
|
This method is the most common way to achieve a usable freebase product. Though it is advisable to keep everything completely free of moisture in this process, impurities carried through by moisture are not dangerous and merely effect weight.
|
Crystalline Conversion
| Crystalline Freebase Conversion of DMT | |
|---|---|
|
Though heptane is a bit more toxic and more difficult to evaporate than acetone, it is able to achieve a more pure, hard, crystalline product. The only likely potential impurity--assuming proper decanting--is residual heptane.
|
Nontoxic Freebase Spice Conversion
Nontoxic Hasty Freebase Spice Conversion
| Nontoxic Hasty Freebase Spice Conversion[1] | ||||||
|---|---|---|---|---|---|---|
|
This method is the most expedient and simplest method of freebase conversion.
|
Nontoxic STB Freebase Conversion of DMT
| Nontoxic Freebase Conversion of DMT[2] | |
|---|---|
|
Though completely nontoxic, this method is reportedly difficult for achieving a manageable product.
|
STB Conversion
This conversion is simplistic in that it almost exactly resembles the methods used in STB Techniques, though it is significantly simpler in that it involves less material, fewer impurities, and does not require a strong base.
| Materials Required | |
|---|---|
| Source Material: |
|
Solvents:
|
|
Reagents/Desiccants:
|
Procedure for STB Freebase Conversion of DMT:
- Dissolve DMT Fumarate in an adequate amount of water.
- No reaction occurs at this point.
- Add a concentrated solution of weak base until total precipitation is observed by the cloudiness of the solution.
- The fumaric acid undergoes reaction with the base, effectively neutralizing the acid and freeing the product in its pure alkaloid form, or freebase.
- Stir in NPS thoroughly and allow to separate from the aqueous solution.
- The more contact allotted between the product and the NPS, the greater the saturation.
- Collect the top layer of NPS, being careful not to allow any aqueous contamination.
- Aqueous contamination may result in an impure product or may disrupt subsequent crystallization.
- Repeat steps 3-4 with fresh or unsaturated NPS until the aqueous solution is exhausted to satisfaction.
- Three washes is generally considered sufficient.
- See Crystallization in order to render crystalline freebase product.
Further Elaboration and Technical Support
Administration
Leaf Enhancement
Vaporization
The vaporization method of administration pertains to the use of pure freebase product with no additional material. This method generally makes use of heating an apparatus that is intended to distribute the heat to the product until it reaches the point of vaporization and can be inhaled.
Glass-Pipe Vaporizers
Glass-pipe vaporizers are glass pieces that are meant to be heated directly in order to indirectly disperse that heat into the product. The glass used must be thin enough for the heat to pass through its structure and potentially distribute the heat evenly. The piece must have a chamber within which the product will undergo vaporization. It must be assembled in a manner that will allow for air intake and output: The output being the inhalation nozzle, and the input being a sort of carb.
| the Improvisation of a Glass Pipe Vaporizer | |
|---|---|
The Machine
"The Machine" is essentially a glass vaporizer in which heat is meant to distribute through the pipe rather than across the glass. It utilizes a metal mesh plug inside of the pipe, on which the product is to be placed, melted and vaporized. The mesh acts both as a screen and a heat-sink, simultaneously allowing for the even heating of the product, protection from the naked flame, and prevention from inhaling unvaporized particulates. The manner of heating is likely to be conduction, as the product is known to run from the heat across the mesh until completely vaporized; however, most designs cause the product to simply run into more heat, resulting in continuous vaporization.
This method of vaporization includes the standard variations—which are essentially the simple combination of a vaporizing bowl and a vapor chamber with an inhalation nozzle—and the bubbler variations in which the vapor passes through a water heat-sink before reaching the vapor chamber and inhalation nozzle.
Material Considerations:
- Copper may gradually turn black due to the formation of Copper(II) oxide (CuO), an inert solid. This reaction and its products are not detrimental to the process of administration.
| the Improvisation of the Standard Variation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Machine-Style Bubbler Piece
| NOTE | |
|---|---|
| Theses variations can apply to the use of a small bubbler pipes, bong-style bubblers, or improvised bubblers, though smaller bubblers are often found most preferable. The water in the bubbler acts only as a heat-sink and will not absorb a significant, if any, amount of product, as the freebase product is not soluble in water; however, the use of ice in cooling should be forgone, as it may cause the premature precipitation of product within the chamber. |
| the Improvisation of the Bubbler Bowl Variation of "The Machine"[8] | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| the Improvisation of the Machine-Style Bubbler Stem[9] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Further Elaboration and Technical Support
Effective Use and Maintenance
As the term indicates, vaporizers are intended to vaporize, not to burn product, as such, the product should never come into contact with the flame or be overly heated to the point of burning. Generally, a vacuum must be generated in order to direct the heat through the product and to direct the vapor into the chamber. Which should not be allowed to sit for too long, as it may begin to precipitate within the chamber. Every toke should be held in the lungs as long as necessary for the vapor to be completely absorbed, as no vapor should be exhaled.
Heat-Source:
- Though many prefer a butane torch for expedient heating, and though many manners of vaporizer demand its use, a standard lighter will produce adequate heat for vaporization. A standard lighter is generally ideal for vaporizers that generate a strong enough vacuum to pull the flame toward the heat sink.
Common Methods of Inhalation:
- Some prefer to vaporize their entire dose before inhaling so as to administer one strong toke. This method can be quite harsh and difficult on the lungs and throat and may induce coughing. However, this is reportedly the most intense method of administration, almost always inducing a "breakthrough" if held in the lungs for adequate absorption.
- Others prefer to administer one dose within multiple tokes, which is reportedly slightly longer-lasting and much easier on the lungs and throat though possibly less intense. Usually the amount of tokes taken depends on the intended depth of the experience. In many cases, three is found to be more than adequate and four is said to be a "breakthrough" dose. Any subsequent tokes to the first toke must occur within the first minute, due to both tolerance and lack of motor skills building up rapidly
Cleaning the Apparatus:
- The apparatus used for vaporization can be cleaned using the same solvents used for extraction. However, volatility and toxicity concerns should be a strong consideration in choosing the proper solvent.
- It is preferable to choose a solvent that evaporates quickly and cleanly and able to dissolve a broad range of products, as oxidization is likely to have occurred.
- Residual solvent residue may be hazardous, as the user may inhale harmful fumes or potentially ignite the solvent.
- The residual product dissolved by the cleaning solvent may be salvaged by appropriate methods of crystallization. However, it is likely to contain a variety of inactive constituents, so proper purification measures would be advisable.
Concerns Regarding the Experience
- The primary effects of smoked DMT, induced within minutes of administration, last for about 5-10 minutes and are characterized by strong visual hallucination, a strong psychedelic quality, and minor auditory hallucination, while the secondary effects of a much more mild character may last for up to an hour.
- Tolerance is reportedly induced rapidly and often dissipates rapidly, though repeated or high doses may induce a slightly longer term tolerance. Some believe it is best to wait at least one hour between doses.
- The experience of smoking DMT is said to be quite overwhelming. It is often necessary for the user to have a "sitter" nearby to tend to the smoking apparatus so as to not risk the possibility of the user damaging it or accidentally inflicting burns. It can also be temporarily debilitating, so the user may be advised to refrain from any physical activity.
- Set and setting are of the utmost importance, as the user is often rendered temporarily vulnerable and emotionally fragile. The user must determine the appropriate conditions in which administration is to take place but is advised to initially seek out a setting containing minimal stimuli.
- It may also behoove the user to seek out a more experienced sitter for guidance and support. Often this is unavailable, and as such, it would be more advisable for the user to perform the rite in solitude. An inexperienced sitter may inadvertently induce anxiety in the user out of negligence or unnecessary concern.
Further Elaboration and Technical Support
Potentiation
- The effects of DMT, as with most psychedelic substances, can be lengthened, strengthened, altered, or otherwise potentiated through the use of various psychoactive or bioactive compounds. The potentiation of DMT is actually the oldest method of administration, as its necessarily potentiated oral administration serves as the compound's longest known history of use. DMT is generally considered to be orally inactive without some form of potentiation, such as a harmaloid preparation. DMT's potentiation is not limited to oral use, however, as many of the same potentiating agents may be used in conjunction with vaporized or otherwise administered DMT.
Harmaloids
Harmaloids
Oral Administration of DMT
Pharmahuasca
Pharmahuasca is a pharmaceutical version of the entheogenic brew ayahuasca. Traditional ayahuasca is made by brewing the MAOI-containing Banisteriopsis caapi vine with a DMT containing plant, such as Psychotria viridis. Pharmahuasca refers to a similar combination that uses a pharmaceutical MAOI instead of a plant.
- A general rule for the dose taken of DMT in a capsule is NO MORE THAN 1mg per pound of body weight.
- This is assuming that approx 100mg MAOI is taken first.
- Take one MAOI capsule (100mg) wait ten-twenty minutes
- Take one DMT capsule containg half of the dose (for 150lbs. bodyweight, no more than 75mg DMT)
- Wait ten minutes, this will reduce the likelihood of vomiting.
- Take one DMT capsule containing the second half of the dose.
- Experiment with how much spice SWIM likes to use and find what's right for SWIM.
WARNING
- This method of administration can easily get out of hand by believing a small change in the weight of the dose will have little effect, the above dosage is considered to be a high one. Do not attempt to operate machinery during the trip. Do none of these things, after all SWIM will probably be lying on their back for a couple hours.
Oral Administration
Pharmahuasca
Pharmahuasca is a pharmaceutical version of the entheogenic brew ayahuasca. Traditional ayahuasca is made by brewing the MAOI-containing Banisteriopsis caapi vine with a DMT containing plant, such as Psychotria viridis. Pharmahuasca refers to a similar combination that uses a pharmaceutical MAOI instead of a plant.
- A general rule for the dose taken of DMT in a capsule is NO MORE THAN 1mg per pound of body weight.
- This is assuming that approx 100mg MAOI is taken first.
- Take one MAOI capsule (100mg) wait ten-twenty minutes
- Take one DMT capsule containg half of the dose (for 150lbs. bodyweight, no more than 75mg DMT)
- Wait ten minutes, this will reduce the likelihood of vomiting.
- Take one DMT capsule containing the second half of the dose.
- Experiment with how much spice SWIM likes to use and find what's right for SWIM.
WARNING
- This method of administration can easily get out of hand by believing a small change in the weight of the dose will have little effect, the above dosage is considered to be a high one. Do not attempt to operate machinery during the trip. Do none of these things, after all SWIM will probably be lying on their back for a couple hours.
Jungle Spice
Jungle spice is one of many names that have been applied to an intriguing non-DMT alkaloid fraction that can be isolated from much of the commercially available Mimosa root bark (See V. Botanical Confustication).[10],[11],[12] In the most general terms, it is the alkaloid fraction obtained from the aqueous basic phase of an extraction by pulling with xylene or toluene after DMT ceases to be pulled by an aliphatic hydrocarbon solvent (naphtha, heptane, etc.). This product will almost always contain some N,N-DMT in addition to the more mysterious alkaloids; some extractors choose to remove the DMT in a hot naphtha wash to obtain a pure "jungle" experience, while others use the jungle/DMT mixture as it is.
There is a great deal of ambiguity surrounding jungle spice, owing to a wide array of factors. First and foremost, there appears to be a great diversity of compounds which can be isolated by extracting the aqueous basic phase with xylene or toluene.[13],[14] Which compounds are actually isolated depends on some several of the following factors: the source and botanical identity of the root bark, the conditions of cultivation/harvest, and various pH, temperature, and airflow considerations throughout the extraction process.[15],[16],[17],[18] About all that is certain about it at this point is that it contains some psychoactive material that isn't N,N-DMT.
There has been a lot of speculation going around that this compound may be yuremamine, the novel phytoindole isolated from Mimosa Tenuiflora and characterized in 2005.[19],[20],[21] Looking at the evidence, this scenario appears exceedingly unlikely based on yuremamine's known instability to base and speculated instability to heat.[22],[23] It can't yet be ruled out completely, but there remains a substantial body of evidence against this identification. Until an LC-MS of jungle spice emerges with a molecular ion at 477.1 m/z, I think it's safe to assume that yuremamine is not the red alkaloid that has been isolated by home extractors.
Links
- Summary of jungle spice analytical work
- "Jungle Spice" - Mystery Alkaloids of Mimosa Root Bark at the DMT-Nexus
- Entropymancers "Jungle Spice" - Mystery Alkaloids of Mimosa Root Bark.pdf
Further Potentiation
Appendices
Storage Concerns
- DMT should be stored in a cool and dry place, preferably in a sealed container.
Discussion of Oxidization
Overview
Conversion
Administration of DMT N-Oxide
Analogues
5-MEO-DMT
Bufotenine
References
- ↑ Amor_fati's_Approach_to_Freebase_Conversion_of_DMT#Nontoxic_Conversion
- ↑ DMT Fumarate to DMT Freebase
- ↑ "The_(mini)_Machine"_Step_by_Step_Guide
- ↑ q21q21's Test-Tube Machine
- ↑ Reintroducing My Alternative to "The Machine" by amor_fati
- ↑ New stealth bargan vapour pipe by deweeb
- ↑ My Machine by Aoutiv
- ↑ Posts Regarding Construction and Operation of a Machine-Style Bubbler Bowl by WSaged[1][2][3][4]
- ↑ The Mini-Machine Bubbler Stem
- ↑ Doctorcito (@ Ayahuasca Forums). 2006. "Yuremamine: Solving the Mystery of Jurema Preta?" http://forums.ayahuasca.com/phpbb/viewtopic.php?t=9912
- ↑ DonPeyote (@ Drugs-Forum). 2006. "Jungle DMT?" http://www.drugs-forum.co.uk/forum/showthread.php?t=26468
- ↑ Lemmiwinks (@ Entheogen.com Forums). 2007. "Red Spice?" http://www.entheogen.com/forum/showthread.php?t=11191
- ↑ Fuego (@ DMT-Nexus Forums). 2007. "Doing xylol extraction." http://www.dmt-nexus.me/forum/default.aspx?g=posts&t=398
- ↑ Marsofold (@ The Nook Forums). 2005. "Extraction Of An Alternate Alkaloid From Mimosa." http://www.thenook.org/forum/index.php?showtopic=39278
- ↑ Doctorcito (@ Ayahuasca Forums). 2006. "Yuremamine: Solving the Mystery of Jurema Preta?" http://forums.ayahuasca.com/phpbb/viewtopic.php?t=9912
- ↑ DonPeyote (@ Drugs-Forum). 2006. "Jungle DMT?" http://www.drugs-forum.co.uk/forum/showthread.php?t=26468
- ↑ Noman (@ DMT-Nexus Forums). 2006. "Dark DMT - the Other Alkaloid." http://www.dmt-nexus.me/forum/Default.aspx?g=posts&t=90
- ↑ Noman (@ The Lycaeum Forums). 2006. "Dark DMT." http://forums.lycaeum.org/index.php/topic,17215.0.html
- ↑ Vepsäläinen JJ, Auriola S, Tukiainen M, Ropponen N, Callaway JC. 2005. "Isolation and characterization of yuremamine, a new phytoindole." Planta Med. 71(11):1053-7
- ↑ Marsofold (@ Drugs-Forum). 2005. "The Other Alkaloid In Mimosa Hostilis." http://www.drugs-forum.co.uk/forum/showthread.php?t=13507
- ↑ Noman (@ DMT-Nexus Forums). 2006. "Dark DMT - the Other Alkaloid." http://www.dmt-nexus.me/forum/Default.aspx?g=posts&t=90
- ↑ Vepsäläinen JJ, Auriola S, Tukiainen M, Ropponen N, Callaway JC. 2005. "Isolation and characterization of yuremamine, a new phytoindole." Planta Med. 71(11):1053-7
- ↑ Noman (@ DMT-Nexus Forums). 2006. "Dark DMT - the Other Alkaloid." http://www.dmt-nexus.me/forum/Default.aspx?g=posts&t=90