Chilled Acetone with IPA and Naphtha
Contents
Headline text
Introduction 🙏
Min Poly stands for Minimun Polymer.
In this technique (TEK), DMT polymers (also called DMT aggregates or DMT oligomers) are minimized. First, any natural DMT aggregates are broken down in an acidic pressure cooking process using vitamin C. Secondly, during the basing step, ionic strength, alkalinity, and DMT concentration are kept low to avoid DMT (re)polymerization.
Thanks to Dragonrider for sharing his excellent pressure cooking experimental result, to Benzyme for showing MS evidence of DMT aggregates, and to Jees, Downwardsfromzero and Loveall for their contributions to this process.
Safety ⛑️
Review NaOH[1] and naphtha [2] safety information. Verify solvent MSDS purity, plastic compatibility, and clean evaporation.
Never have solvents near an open flame.
Following this advice does not guarantee safety. It is up to each adult individual to make their own decision.
Materials🛒
Consumables👩🌾
- ~800ml water (for canning jar)
- ~A few liters of water (for pressure cooker)
- 25g ascorbic acid (vitamin C)
- 50g root bark
- ~400ml naphtha
- 25g of NaOH
Equipment🏺
- Two 1-quart canning jar with new lids
- Scale
- Mixing tool (e.g. butter knife)
- Pressure cooker
- Pippette
- Shallow baking dish
- Fan
- Plastic wrap
- Freezer
- Razor blade
Process Overview 👀
- Prepare canning jar with root bark, water, and vitamin C
- Pressure cook for several hours
- Basify with naphtha present
- Pull, freeze precipate, and collect
Detailed Process 📜
Prepare Canning Jar 🥫
Mix, water, vitamin C and root bark in a quart jar. Leave water level <1 inch from the top of the jar for proper headspace canning
Pressure Cook 🔥
Pressure cook jar at 15 PSI for 4 hours. Follow manufacturers canning instructions to avoid liquid loss and/or a cracked jar.
Tips: (1) Jar lid needs to be new and screwed on snug to avoid liquid loss, but not too tight to avoid cracking, (2) have a raised inset and enough water in the pressure cooker for a long run (3) prepare jar for pressure by boiling for 10 minutes before adding pressure cooker nozzle weight, and (4) do not release pressure when run is complete (allow it to slowly drop passively).
If jar breaks or liquid is lost, simply reduce the contents of the pressure cooker over heat and combine them into a single jar. This is added work, so try to avoid it by following canning instructions precisely.
Pull 👩🔬
Allow pressure cooker jar to cool to room temperature. Add ~100ml of naphtha (almost completely filling the jar) and shake. Add lye and shake vigorously for 5 minutes. Solution will warm up slightly as lye dissolves, so release pressure from warm naphtha. Heat in warm water bath to 40-50C and shake for another 5 minutes (again, remember to release pressure).
Rest solution in the water bath until naphtha layer separates (~30 minutes). If separation is not complete, mix in another 5g of lye and try again.
Remove naphtha with a pipette or turkey baster into a second quart jar. It is ok if a few drops of watery extract come through (they will be decanted later).
Add another ~100ml of naphtha to the first jar. Get solution back to 40 to 50C using a warm water bath. Shake for 5 minutes (remember to release any pressure buildup). Rest until layers separate, and pippette naphtha into the second jar. Perform this additional pull step 3 times (total of 4 pulls)
Crystalize ✨
Carefully decant naphtha in second jar to a shallow baking dish. Do not allow any watery extract or particles to come through.
Evaporate solvent until slightly cloudy with the help of a fan in a well ventilated area. Note that minimum polymer extracts tend to cloud less than other TEKs. It is OK if no cloudiness is present after 50% naphtha has evaporated, and the extract can be moved to the freezer at this point. If evaporation is skipped, yield will be lower but more naphtha (containing some DMT) will be available for reuse in future extractions. Re-used naphtha does not need to be pre-evaporated before freezing since it is already saturated at the freezing temperature.
Cover shallow baking dish with plastic wrap and place in the freezer for at least 24 hours so DMT crystals form.
Decant naphtha off crystals, and immediately dry baking dish with the help of a fan. Keep dish tilted in the same direction after decanting to avoid warm naphtha residue from washing over crystals.
Once dry, scrape up dry xtals with razor blade. Avoid bottom edge if oily. This is the final product.
Collect 💖
Catch loose crystals in a coffee filter. Rinse crystals on wall and filter with fresh ethyl acetate (~2-3x until off color is removed). Collect crystals stuck on the jar walls by dissolving them in warm water, evaporating in a shallow dish, and scraping up dry crystals. Combine with the collected crystals from the filter to obtain the final product (Fig. 5).
Yield depends on the cactus and is usually between 0.2% to 2% with ~1% being common[3]. The precise ratio of mescaline and citrate in the precipitate is not known and is under investigation (see development notes below). Preliminarily data indicate the salt made my this process is ~60% as strong as mescaline HCl and consistent with the salt form (MesH)H2Cit. Approximate oral dosage recommendations for mescaline HCl can be found elsewhere[4].
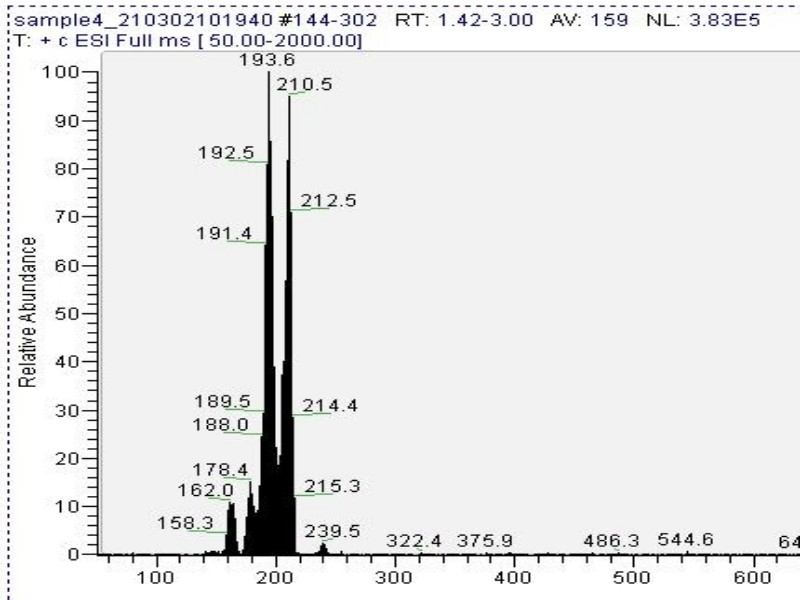
Mass spectrometry (MS) results from solaris analytical[5] indicate the product is very clean mescaline (Fig. 6).
Reclaim Solvent 💚
Reusing solvents is encouraged[6] at the DMT nexus.
Simply reuse freeze precipitated naphtha as-is. Re-used naphtha is saturated with DMT at freezer temperature and pre-freezer evaporation is not needed. Easy 😊
Frequently Asked Questions ❓
Q: That's a lot of hypothesis you got there. Have any experimental evidence consistent with them?
A: Yes. Benzyme's MS, along with more direct polymerization and de-polymerization experiments. As far as we know experiments are consistent with the hypotheses listed. The community is welcome to update this Wiki entry as more evidence arises, especially if any of the hypotheses are disproved (thank you).
Q: What's so special about citric acid?
A: See the development note below.
Appendix: Development Notes 🔬
Hypotheses 🤔
This technique (TEK) hypothesizes that:
- Not all of the DMT is in the plant in monometer form, some of it is in macro-molecule aggregates (sometimes called DMT polymers or DMT oligomers)
- DMT can also form aggregates during the basing step in high alkaline, high ionic strength, and high DMT concentration conditions
- DMT aggregates are more difficult to dissolve in naphtha compared to the DMT monomer and require heat. During freeze precipitation or evaporation from naphtha, DMT aggregates quickly cloud and precipitate as yellow/orange/red oils, while monomers cloud later and slowly form white crystals.
- DMT monomer benefits:
- Improved partition coefficient (fewer pulls for good yield)
- Easier to handle and dose precisely
- Low and consistent vaporization temperature, ideal for newer electronic vaporization devices with precisely tuned temperature settings
- Visibly unique upon crystalization, making confident identification easier
- Easier to complex with HPBCD for sublingual administration
- Other properties
- It is unknown if DMT monomer has better bioavailability for oral or rectal administration. In principle, stomach acid should be able to break down DMT into its monomer form
- There is no expected benefit for DMT monomer over DMT aggregates for torch vaporization
Strategy ♟️
The strategy of this TEK is to break down both DMT aggregates and plant material, while minimizing DMT re-aggregation during the basing step.
Agressive alkaline concentration conditions are avoided. While these type of processes can break down plant material, their downside is that they don't break down natural DMT aggregates and can even increase the degree of polymerization.
Fortunately, DMT aggregates can break down in acidic conditions. Therefore, to simultaneously break down DMT aggregates and plant material, a long acidic pressure cooking step is used (described before by for example Dragonrider). Vitamin C is chosen as the source of acid due to its good experimental performance, but other acids could also work. Subsequently, relatively gentle ionic strength (no added salt), alkaline pH (no excess lye beyond emulsion breakdown), and low DMT concentration (<0.5%) conditions are used to minimize any DMT re-aggregation. Naphtha is also present during the basing step to minimize the time DMT spends in the base when it is at its highest initial concentration.
Vitamin C 🍊
Experimentally, Vitamin C produced better results compared to acetic and citric acids. Vitamin C is biologically active as a mild antioxidant and reducing agent and can pass through cell membranes. During pressure cooking, Vitamin C breaks down into several other acids such as dehydroascorbic acid, diketogulonic acid, xylonic acid, threonic acid and oxalic acid. It could be that by being exposed to multiple different acids DMT de-polymerization is improved by complimentary mechanisms.
Other acids may also work, and the kitchen alchemist is encouraged to report on any new experimental results (both positive and negatives).
Cloudiness 🌫️
DMT monomer does not readily form clouds in naphtha. Compared to other extractions that do not minimize polymer, clouds for later in the evaporation process and are not as opaque. This is a good sign and not a cause for concern. It is OK to freeze precipate before clouds are observed after reducing the solvent volume.