CIELO
Contents
Introduction 🙏
CIELO stands for Crystals In Ethyl-acetate Leisurely Over-the-counter.
In this technique (TEK), cactus lime paste (Fig. 2) is extracted with ethyl acetate (Fig. 3). Mescaline citrate is precipitated with citric acid (Fig. 4) and collected (Fig. 5).
Thanks to everyone who contributed to this process: someblackguy, Benzyme, shroombee, Metta-Morpheus, Downwardsfromzero, Kash, grollum, Mindlusion, Doubledog, Dreamer042, Loveall, and others.
Safety ⛑️
Review ethyl acetate's[1] and citric acid's[2] safety information. Verify solvent MSDS, plastic compatibility, and clean evaporation.
Following this advice does not guarantee safety. It is up to each adult individual to make their own decision.
Materials 🛒
- French press (or similar ensemble)
- 300g water
- 25g lime
- 100g dry cactus powder
- 1qt ethyl acetate (also sold as "MEK substitute")
- Coffee filters, support basket, and funnel
- Quart jar
- 5g of citric acid (15g is another option)
- Washing soda (for solvent reclaim)
Process 📜
Paste 🌵
Mix water and lime first to make milk of lime in french press (or quart jar if french press is not available) Without giving lime time to settle, and cactus to french and mix well for 10 at least minutes to a fluffy smooth paste (Fig. 2). When the mixing is performed properly a change in the paste texture occurs. In case of doubt mix more.
Extract 👨🏾🔬
Cover paste with freezer ethyl acetate, mix well for 60s, allow to rest for 120s and decant into quart jar through a coffee filter. Do not squeeze with the french press, its purpose is to only hold back the paste from falling into the filter. Repeat until quart jar is full (~5x).
Inspect extract for droplets or particles. If present, remove them. Extract needs to be clean (see Fig. 3). To be sure extract is clean, one extra pass through a fresh coffee filter is recommended.
Crystalize ✨
Drop citric acid into extract and let it slowly dissolve by diffusion over time. Clouds form, followed by mescaline citrate crystals. Crystals can have different shapes and sometimes also stick to the wall looking transparent. Allow crystalization to complete undisturbed (~24 hours).
One crystalization option proposed by shroombee is to use 15g of citric acid and continuous magnetic stirring (or continuous shaking). This produces a fast crystalization and minimizes crystals that are stuck to the wall. Crystals may be smaller with this approach, but the vast majority of them will still be caught by a filter in the next step.
Collect 💖
Catch loose crystals in a coffee filter. Rinse crystals on wall and in filter with fresh ethyl acetate (~2-3x until off color is removed). Collect crystals stuck on the jar walls by dissolving them in warm water and evaporating. Combine with the collected crystals from the filter to obtain the final product (Fig. 5).
Yield depends on the cactus and is usually between 0.2% to 2% with ~1% being common[3]. By weight, mescaline citrate is equivalent to mescaline sulfate dihydrate and 90% as strong as MescalineHCl. Oral Dosage recommendation[4]
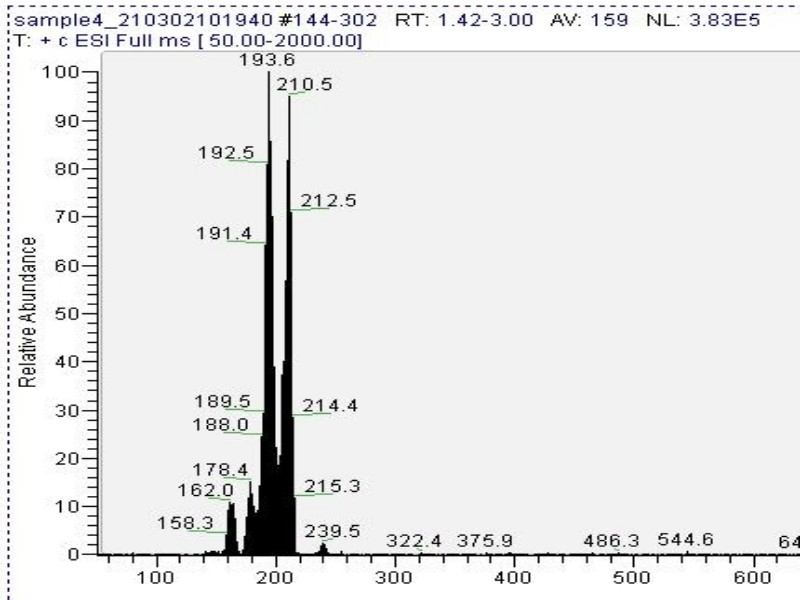
Mass spectrometry (MS) results from solaris analytical[5] indicate the product is very clean mescaline (Fig. 6).
Reclaim Solvent 💚
Reusing solvents is encouraged[6] at the DMT nexus.
Wash spent extract with sodium carbonate saturated water shaking vigorously (emulsions do not form). Filter any excess sodium carbonate and remove water layer. Freeze and filter out ice crystals. Optionally, remove color soaking over activated carbon for several days an filter.
Appendix: Development Notes 🔬
Paste 🌵
No improvements were seen with longer basing time, microwaving, drying, or increasing the ionic strength.
Paste made with sodium carbonate saturated water congeals over time and requires long solvent soaks which are darker and don't crystallize to large loose crystals (small sticky crystals were obtained).
Extract 👨🏾🔬
Longer/warmer pulls resulted in darker extract, smaller crystals, solvent paste absorption, congealing of paste, and no yield benefit.
Chemically drying the extract had no benefits.
An additional long room temperature pull on the TEK's spent paste only yielded 4mg of very small crystals, indicating the chilled pulls are efficient.
Crystalize ✨
During crystallization, every 233mg of citric acid (H3Cit) react with free base mescaline (Mes) to form to 1g of mescaline citrate (or slightly more if a hydrate is precipitating):
Excess citric acid shifts the precipitation reaction to the right (Le Chatelier's principle), helping overcome water and plant material. There is a lot room for excess citric acid in solution since its solubility is 50mg/g in ethyl acetate. The TEK recommends ~5mg/g but since cacti and pull techniques can vary, users may find other values work better for their specific situation (in one example with whole cactus powder 20mg/g was used [7]).
Several factors can make crystals smaller: Reusing ethyl acetate, longer/warmer pulls, higher citric acid concentration, mechanical agitation, and other potential variables. Small crystals can look like a fine powder. Potency does not seem affected by the crystallization appearance, and a powdery precipitate is not a problem unless it becomes difficult to decant/filter.
After the initial crystallization, adding more citric acid and/or moving the extract to the refrigerator did not result in any more precipitation. Moving the extract to the freezer produced ice crystals.
Other dry organic acids could work. Fumaric, Malic, Tartaric, Ascorbic, Succinic, etc can be tested in future investigations.
Collect 💖
Washing crystals in a filter appears to wick away plant colors and superior to decanting.
The washed crystals in the filter can also be dissolved in warm water along with any wall crystals. This will give then final product a uniform appearence.