Chilled Acetone with IPA and Naphtha
Contents
Headline text
Introduction 🙏
Min Poly stands for Minimun Polymer.
In this technique (TEK), DMT polymers (also called DMT aggregates or DMT oligomers) are minimized. First, any natural DMT aggregates are broken down in an acidic pressure cooking process using vitamin C. Secondly, during the basing step, ionic strength, alkalinity, and DMT concentration are kept low to avoid DMT (re)polymerization.
Thanks to Dragonrider for sharing his excellent pressure cooking experimental result, to Benzyme for showing MS evidence of DMT aggregates, and to Jees, Downwardsfromzero and Loveall for their contributions to this process.
Safety ⛑️
Review ethyl acetate[1] and citric acid[2] safety information. Verify solvent MSDS, plastic compatibility, and clean evaporation.
Never have solvents near an open flame.
This TEK is food safe if food grade materials are used.
Following this advice does not guarantee safety. It is up to each adult individual to make their own decision.
Materials🛒
Consumables👩🌾
- 200g water
- 20g washing soda
- 100g ground rue seeds
- ~3/4 qt ethyl acetate (also sold as "MEK substitute")
- 150ml of vinegar
- 30g of NaCl
- Additional washing soda and pH paper (to wash solvent for reuse)
Equipment🏺
- Coffee grinder
- French press for mixing and pulling rue with ethyl acetate (a quart jar also works but is not as easy as the French press)
- Microwave
- Quart jar to collect pulls and precipitate harmala HCl
- Coffee filters, support basket, and funnel
- Kitchen scale to weigh materials
- Pot and source of heat to warm water
Process Overview 👀
- Make wet alkaline microwaved rue paste
- Pull with ethyl acetate
- Salt with vinegar/NaCl solution
- Rest in fridge overnight for harmala HCl xtals to form
- Collect xtals, wash, and squeeze dry
Detailed Process 📜
Paste 🌵
Dissolve 20g of washing soda in 200g of water in a french press (or a quart jar). Ground rue seeds and add then to the alkaline solution a, stirring thoroughly. Microwave in short runs of 2-3 minutes stirring between runs until the paste has no running water (~100g of water removed).
Extract 👨🏾🔬
Cover paste with two fingers works of ethyl acetate (~100g) and stir for a few seconds. Loosely cover and heat in a hot water bath brining ethyl acetate to a boil (do not seal container to avoid pressure buildup). Extract with boiling EA for least 10 minutes, stirring every couple minutes or so (do not have any open flame sources and work in a well ventilated area). Decant into quart jar. Do not squeeze with the French press, its purpose is to only hold back the paste from falling into the filter. Squeezing can push unwanted plant material or water into the extract. Repeat adding ethyl acetate, heating, stirring, and decanting until quart jar is ~3/4 full. This will be about 5 total pulls.
Rest the combined extract for at least an hour and then inspect for droplets or particles. If present, allow extract to rest until no more debris form and remove them with a filter.
Room temperature extract option: With this option the paste is extracted at room temperature over 24 hours and shaken throughout the day. This extraction is more passive and slower, but yields are similar[12].
Crystalize ✨
Dissolve 30g of NaCl into 150 grams vinegar. Add this solution to the combined EA pulls. White clouds will form in ethyl acetate, shake to clear clouds and move harmala salts into the water layer. Rest for 10 minutes and if a red oil forms filter or decant it off. Move salted extract to the fridge for 24 hours. Harmine and harmaline HCl crystals should form in the aqueous layer.
Collect 💖
Catch loose crystals in a coffee filter. Rinse crystals on wall and filter with fresh ethyl acetate (~2-3x until off color is removed). Collect crystals stuck on the jar walls by dissolving them in warm water, evaporating in a shallow dish, and scraping up dry crystals. Combine with the collected crystals from the filter to obtain the final product (Fig. 5).
Yield depends on the cactus and is usually between 0.2% to 2% with ~1% being common[3]. The precise ratio of mescaline and citrate in the precipitate is not known and is under investigation (see development notes below). Preliminarily data indicate the salt made my this process is ~60% as strong as mescaline HCl and consistent with the salt form (MesH)H2Cit. Approximate oral dosage recommendations for mescaline HCl can be found elsewhere[4].
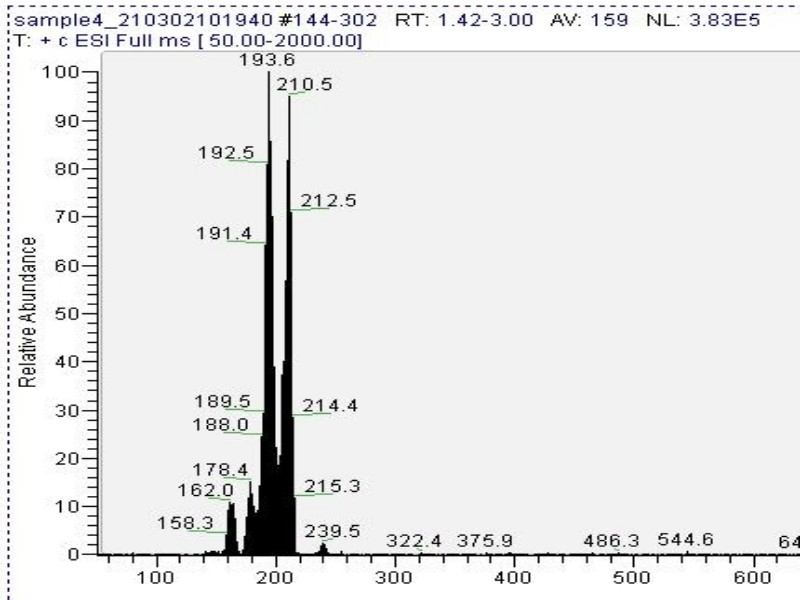
Mass spectrometry (MS) results from solaris analytical[5] indicate the product is very clean mescaline (Fig. 6).
Reclaim Solvent 💚
Reusing solvents is encouraged[6] at the DMT nexus.
Used solvent from the microwave paste process saturates with tan color and can be reused many times. This process is recommended for reuse. The non microwaved paste seems to load the solvent with chlorophyll indefinitely, making it black/opaque and difficult to reuse.
Wash spent extract with sodium carbonate saturated water (35% by weight). About 1/5 of the solvent volume as saturated water is enough. Shake vigorously (emulsions do not form). CO2 bubbles may be visible during citric acid neutralization. Keep an eye on any bubbles and release any pressure buildup regularly. Neutralization can be optionally verified with pH paper. Filter any excess sodium carbonate/citrate and remove the water layer. Tan solvent can be reused on another microwaved paste.
If planning to extract cold, chilled reused paste will have ice crystals that can be removed with a filter before the cold extraction.
Frequently Asked Questions ❓
Q: Why does the TEK have so many options. I feel like I'm choosing my own adventure!
A: The options exist to help with crystallization for variable starting plant material. The easiest material to crystalize experimentally seems to be aged pachanoi outer skin powder (aged long enough to change color from green to tan). A more difficult material to crystalize is whole bridgessi powder recently dried and ground. For outer skin pachanoi the simpler path has worked for many without adding any options: no microwave, room temp extraction, no solvent dry with washing soda, and citric acid diffusion. For whole cactus more work may be needed to keep plant material interfering with crystallization by adding all options: microwave paste, cold extraction, citric acid added with continuous magnetic stirrer. Finally, to grow large crystals good options are to microwave the paste and use cold pulls (while allowing citric acid to diffuse slowly). Current general guidelines (as of summer 2021):
- Outer skin pachanoi: Use no options.
- Whole cactus: Use all options, or at least the microwave option.
- Planning to reuse solvent: Use microwave option.
- Max size crystals: Use outer skin powder with microwave paste and cold extraction options. Fresh ethyl acetate is recommended also. To crystallize, drop acid in an isolated quiet room for 12 hours 😉.
Q: Does increasing the basing time increase the yield?
A: No. Shroombee has tested 15 minute, 24 hour, and 72 hour basing times and there was no difference in yield. Other process variables were 8 minutes incorporating milky water with cactus, 6x3 minute pulls, and 15 mg/gram citric acid added with the fast crystallization method. Loveall has confirmed in his experiments that 10 minute and 24 hour basing times produce the same yield. So we assume that any basing time from 10 minutes through 72 hours will produce the same yield. See a detailed explanation in this post.[7]
Q: What’s the difference between adding 5 mg/gram and 15 mg/gram of citric acid in the fast crystalization method?
A: In general, adding more citric acid and aggressively stirring or shaking will force xtals to form faster. However, the xtals will be smaller and more dense. 5mg/g produces fluffier xtals that are more likely to stick to the sides of the container, thus requiring a wash and evaporation process to recover these sticky xtals. 15 mg/g produces smaller, denser xtals that are less likely to stick to the sides of the container. When pouring through a coffee filter, a negligible amount of tiny xtals will drop through the coffee filter.
Q: What is the upper limit of citric acid that can be added to the extract?
A: The solubility of citric acid in ethyl acetate is over 50 mg citric acid per gram of ethyl acetate. Note that plant matter or other unwanted extraction products may affect the solubility. Stay well under 50 mg/gram to ensure no undissolved citric acid is mixed in with the mescaline citrate.
Q: After adding citric acid, I saw clouds followed by precipitation, but the precipitate reminds me of citric acid. How do I know a mescaline salt is precipitating and not citric acid?
A: Citric acid does not precipitate and stays in solution because it is well bellow its solubility limit (50mg/g) in the TEK. The white particles that form from the clouds are salts and not citric acid. A thorough swirl may be needed at the end to make sure all the added citric acid has dissolved. Once it has dissolved it will not come out of solution as citric acid.
Q: After adding citric acid, I saw clouds but didn't get any solid precipitation, what gives?
A: First make sure the TEK instructions were followed, in particular: well mixed paste, short pull times, no squeezing, clean extract free of debris, washing soda dry, citric acid is in range, etc. If nothing is precipitating, bring up the citric acid concentration up to 20mg/g and wait a few days. Check the jar walls, a transparent product may have precipitated there (e.g. this has been reported for whole bridgessi[8]). Whole cactus seems more difficult to precipitate. A microwave paste treatment and colder pulls with ethyl acetate chilled down to 0F grow larger crystals and should be easier to precipitate because they contain less plant material. If all else fails, pulling the extract with water, evaporating, and washing with fresh ethyl acetate should leave behind a potent residue (dose will be less accurate and can be made proportional to starting cactus amount).
Q: Why don't we know the precise salt form and why does it matter?
A: We think it depends on what crashes first as the acid and base react. Work is ongoing to experimentally verify what that is. In practice this affects yield and dosage numbers as the (di) hydrogen salts are less potent by weight. Update: Early data indicates the dihydrogen salt is forming in this TEK (see below).
Appendix: Development Notes 🔬
Hypotheses 🤔
This technique (TEK) hypothesizes that:
- Not all of the DMT is in the plant in monometer form, some of it is in macro-molecule aggregates (sometimes called DMT polymers or DMT oligomers)
- DMT can also form aggregates during the basing step in high alkaline, high ionic strength, and high DMT concentration conditions
- DMT aggregates are more difficult to dissolve in naphtha compared to the DMT monomer (higher yield with fewer pulls). Upon freeze precipitation from naphtha, DMT aggregates quickly cloud and form yellow/orange/red oils, while monomers cloud later and slowly form white crystals.
- DMT monomer benefits:
- Improved partition coefficient
- Easier to handle
- Low and consistent vaporization temperature, ideal for newer electronic vaporization devices with precisely tuned temperature settings
- Visibly unique upon crystalization, making confident identification easier
- Easier to complex with HPBCD for sublingual administration
- Other properties
- It is unknown if DMT monomer has better bioavailability for oral or rectal administration. In principle, stomach acid should be able to break down DMT into its monomer form
- There is no expected benefit for DMT monomer over DMT aggregates for torch vaporization
Strategy ♟️
The strategy of this TEK is to break down both DMT aggregates and plant material, while minimizing DMT re-aggregation during the basing step.
Fortunately, DMT aggregates can break down in acidic conditions. Therefore, to simultaneously break down DMT aggregates and plant material, a long acidic pressure cooking step is used (described before by for example Dragonrider). Vitamin C is chosen as the source of acid due to its good experimental performance, but other acids could also work. Subsequently, relatively gentle ionic strength (no added salt), alkaline pH (no excess lye beyond emulsion breakdown), and low DMT concentration (<0.5%) conditions are used to minimize any DMT re-aggregation. Naphtha is also present during the basing step to minimize the time DMT spends in the base when it is at its highest initial concentration.
Extract 👨🏾🔬
Tests with longer/warmer pulls resulted in darker extract, smaller crystals, solvent paste absorption, congealing of paste, and no yield benefit.
Chemically drying the extract with a drying agent such as anhydrous CaCl2, MgSO4, Na2CO3 monohydrate (aka super washing soda in the US) had no benefits in preliminarily experimental examples. However, depending on the worker and technique, a chemical dry could reduce water content in the solvent and possibly make crystallization less problematic. After some experience from users, a sodium carbonate wash was recommended in the main TEK. However, this step is optional as the process can still work without it. It was added as an option to increase the robustness of the process.
Crystalize ✨
During crystallization, citric acid (H3Cit) reacts with free base mescaline (Mes) to form to form a mescaline citrate salt. The number of mescaline molecules (n) that react with one molecule of citric acid and precipitate is unknown.
Where n could be 1, 2, or 3. The resulting mescaline citrate salt's strength relative to mescaline HCl is 62%, 81%, and 90% respectively. Preliminarily experimental results indicate n=1[9].
Excess citric acid shifts the precipitation reaction to the right (Le Chatelier's principle), helping overcome water and plant material. There is a lot room for excess citric acid in solution since its solubility is 50mg/g in ethyl acetate. The TEK has options ranging from ~6 to 18mg/g and since cacti and pull techniques can vary, users may find other values work better for their specific situation (in one example with whole cactus powder 20mg/g was used [10]).
Several factors can make crystals smaller: Reusing ethyl acetate, longer/warmer pulls, higher citric acid concentration, mechanical agitation, and other potential variables. Small crystals can look like a fine powder. Potency does not seem affected by the crystallization appearance, and a powdery precipitate is not a problem unless it becomes difficult to decant/filter.
After the initial crystallization, adding more citric acid and/or moving the extract to the refrigerator did not result in any more precipitation. Moving the extract to the freezer produced ice crystals.
Other dry organic acids could work. Malic was tested but did not work as well as citric[11]. Fumaric, Tartaric, Ascorbic, Succinic, etc can be tested in future investigations.
10% sulfuric acid was tested and while some crystals formed, a separate liquid layer also appeared making the process not practical.
Collect 💖
Washing crystals in a filter appears to wick away plant colors and superior to decanting.
The washed crystals in the filter can also be dissolved in warm water along with any wall crystals. This will give then final product a uniform appearence.
References 🗝️
- ↑ Ethyl acetate safety[1]
- ↑ Citric Acid Safety[2]
- ↑ Cactus analysis thread[3]
- ↑ Mescaline Oral Dosage[4]
- ↑ Solaris analytical service[5]
- ↑ On reusing non polar solvent[6]
- ↑ Basing time tests results[7]
- ↑ Whole bridgessi precipitate on jar walls [8]
- ↑ Mescaline Citrate conversion to HCl results[9]
- ↑ Ethyl acetate approach[10]
- ↑ Malic acid test[11]