Difference between revisions of "Chilled Acetone with IPA and Naphtha"
(→Paste 🌵) |
(→Paste 🌵) |
||
| Line 41: | Line 41: | ||
== Paste 🌵 == | == Paste 🌵 == | ||
| − | Mix water and lime in a shallow baking dish. | + | Mix water and lime in a shallow baking dish. Microwave<sub>††</sub> at ~1000W until dry stirring frequently (about 15 to 20 minutes of irradiation time). Grind dry paste, transfer to quart mason jar, and chill I'm freezer (~0°F). |
| − | ††''It is possible to skip the microwave drying, but the extract will contain green plant matter and may not be as easy to crystalize as the dry paste | + | ††''It is possible to skip the microwave drying and mix the paste in the extraction mason jar vessel to begin with, but (1) the extract will contain green plant matter and may not be as easy to crystalize as the dry paste, and (2) ethyl acetate may be harder to reuse'' |
Revision as of 19:32, 23 June 2021
Contents
Introduction 🙏
CIELO stands for Crystals In Ethyl-acetate Leisurely Over-the-counter.
In this technique (TEK), microwaved cactus alkaline paste (Fig. 2) is freeze extracted (~0F) with ethyl acetate (Fig. 3) . Mescaline citrate is precipitated with citric acid (Fig. 4) and collected (Fig. 5).
Thanks to everyone who contributed to this process: someblackguy, Benzyme, shroombee, Metta-Morpheus, Downwardsfromzero, Kash, grollum, Mindlusion, Doubledog, Dreamer042, Loveall, merkin, and others.
Safety ⛑️
Review ethyl acetate[1], and citric acid[2] safety information. Verify solvent MSDS, plastic compatibility, and clean evaporation.
Following this advice does not guarantee safety. It is up to each adult individual to make their own decision.
Materials 🛒
- Shallow baking glass
- Quart mason jars with lids
- Microwave
- Blender
- 300g water
- 25g of lime
- 100g dry cactus powder (outer skin or whole plant)†
- Ethyl acetate (e.g. MEK substitute)
- Coffee filter ensemble (funnel/support basket)
- 1/2 tsp Citric acid
- Washing soda and activated carbon (for solvent reclaim)
†If starting with fresh cacti, store the dark for 3 months, slice into ~half inch disks, dry in oven at lowest heat setting, and grind to a fine powder (keeping spines is ok)[3].
Process 📜
Paste 🌵
Mix water and lime in a shallow baking dish. Microwave†† at ~1000W until dry stirring frequently (about 15 to 20 minutes of irradiation time). Grind dry paste, transfer to quart mason jar, and chill I'm freezer (~0°F).
††It is possible to skip the microwave drying and mix the paste in the extraction mason jar vessel to begin with, but (1) the extract will contain green plant matter and may not be as easy to crystalize as the dry paste, and (2) ethyl acetate may be harder to reuse
Extract 👨🏾🔬
Cover paste with freezer chilled ethyl acetate (~0°F), cap jar, shake vigorously for 60s, and filter off solvent into separate quart jar. Repeat until quart jar is full (~5x).
Inspect extract for droplets or particles. If present, remove them. Extract needs to be free of debris (see Fig. 3).
Crystalize ✨
Drop (do not stir) citric acid into extract. Clouds form quickly, slowly followed by mescaline citrate crystals (Fig. 4). Allow crystalization to complete undisturbed (~12 hours).
Note: If using whole cactus more citric acid may be needed to induce crystallization (up to 1 Tbsp).
Collect 💖
Swirl crystalized extract to knock off crystals from walls and dissolve any remaining citric acid granules. Catch floating crystals in a coffee filter. Repeat with a small amount (~1oz) of fresh room temperature ethyl acetate until all crystals are in the filter and off color is washed (~2-3x). Dry and collect from filter (Fig. 5).
Yield depends on the cactus and is usually between 0.2% to 2% with ~1% being common[4].
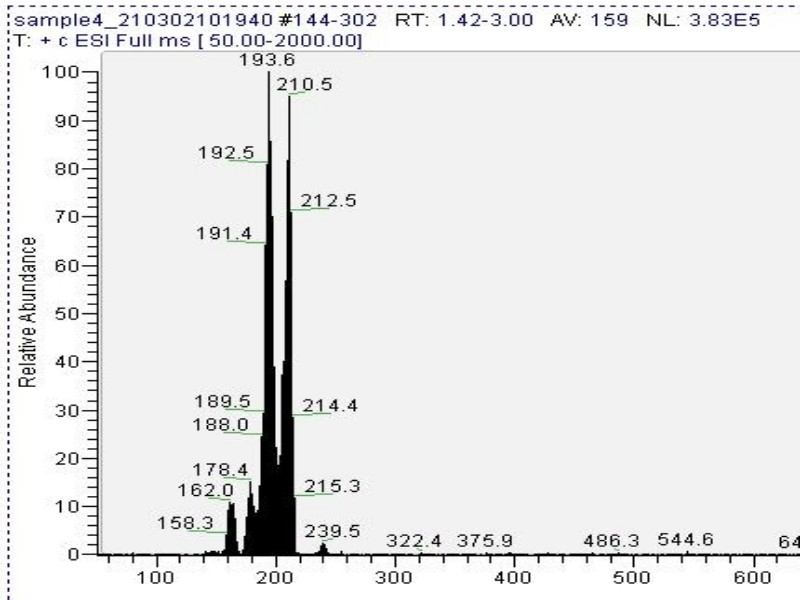
Mass spectrometry (MS) results from solaris analytical[5] indicate the product is very clean mescaline (Fig. 6).
Reclaim Solvent 💚
Reusing solvents is encouraged[6] at the DMT nexus.
Wash extract with sodium carbonate saturated water shaking vigorously (emulsions do not form). Filter any excess calcium carbonate and remove water layer. Freeze and filter out ice crystals. If after multiple reuses a yellow or green color develops it can be removed by resting solvent over activated carbon for several days.
Appendix: Development Notes 🔬
Paste 🌵
Sodium bases and salts (sodium carbonate, sodium hydroxide, and sodium chloride) where tested but made the paste congeal lowering yields. Exclusive use of calcium cations give the paste a "sandy" structure that has more surface area and gives good yields.
In the TEK, two paste treatments on the calcium alkaline paste combine to reduce the amount of plant matter in the extract helping crystallization and purity:
- Microwave Assisted Saponification (MAS[7]) breaks down esters (generally soluble in ethyl acetate which is itself an ester) into carboxylate ions and organic alcohols. An important example is,
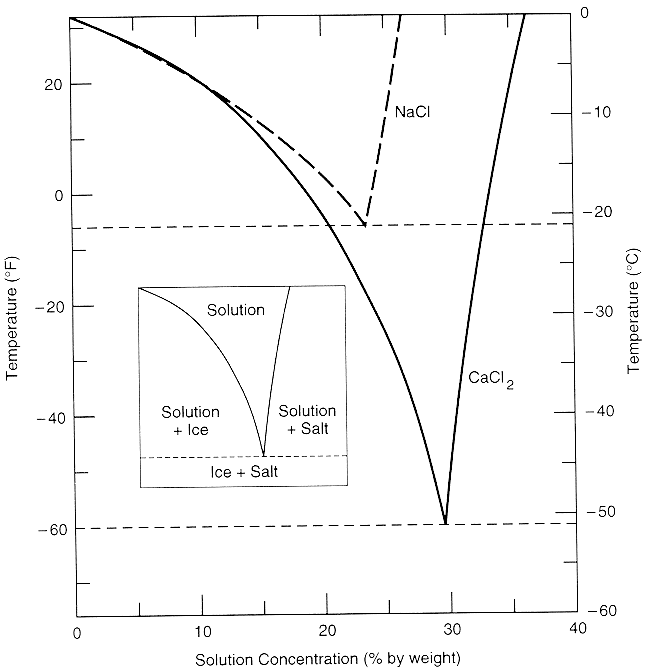
- CaCl2 addition to ~20% water solution enables extraction below ~0F (see Fig. 7). The cold temperature reduces general plant matter solubility in ethyl acetate while mescaline's solubility remains favorable.
Extract 👨🏾🔬
Longer/warmer pulls resulted in darker extract, more difficult crystalization, smaller or sticky crystals, solvent paste absorption, congealing of paste, and no yield benefit.
Chemically drying the extract had no benefits.
An additional long room temperature pull on the TEK's spent paste only yielded 4mg of very small crystals, indicating the chilled pulls are efficient.
Crystalize ✨
During crystallization, every 233mg of citric acid (H3Cit) react with free base mescaline (Mes) to form to 1g of mescaline citrate (or slightly more if a hydrate is precipitating):
Excess citric acid shifts the precipitation reaction to the right (Le Chatelier's principle), helping overcome any water and plant material in the solvent. There is a lot room for excess citric acid in solution since its solubility is 50mg/g in ethyl acetate. The TEK suggests ~3mg/g but since cacti and worker techniques can vary, users may find other values work better for their specific situation (in one specific example with whole cactus powder 20mg/g was used [8]).
Several factors can make crystals smaller: Reusing ethyl acetate, longer/warmer pulls, higher citric acid concentration, mechanical agitation, and other potential variables. Small crystals can look like a fine powder. Potency does not seem affected by the crystallization appearance, and a powdery precipitate is not a problem unless it becomes difficult to decant/filter.
After the initial crystallization, adding more citric acid and/or moving the extract to the refrigerator did not result in any more precipitation. Moving the extract to the freezer produced ice crystals.
Other dry organic acids could work. Fumaric, Malic, Tartaric, Ascorbic, Succinic, etc can be tested in future investigations.
Collect 💖
Washing crystals in a filter appears to wick away plant colors and superior to decanting. Unlike the pulls, warmer ethyl acetate is preferred to wash off plant matter.
The quart jar wash should be done immediately. If any straggler crystals dry in the jar they may stick to the wall. To recover from such a situation, dissolve stuck crystals in hot water, dry in a shallow dish, and scrape.