Difference between revisions of "CIELO"
(→Reuse 💚) |
(→Reuse 💚) |
||
| Line 254: | Line 254: | ||
== Reuse 💚== | == Reuse 💚== | ||
| − | Dark extract can be cleared up with activated carbon (also called activated charcoal). Use | + | Dark extract can be cleared up with activated carbon (also called activated charcoal). Use dustless pellets (typically rinsed with water and dried before use). |
| − | While it is easier to work with a solvent that is | + | While it is easier to work with a solvent that is not dark, a quantifiably benefit of using activated charcoal to decolor the used solvent is not clear. The environmental benefit of regenerating a colorless solvent is in question since ethyl acetate is easy to produce and activated charcoal requires resources to manufacture. |
= References 🗝️= | = References 🗝️= | ||
<references/> | <references/> | ||
Revision as of 13:22, 12 January 2022
Contents
Introduction 🙏
CIELO stands for Crystals In Ethyl-acetate Laizily Over-the-counter. In this process, mescaline from cactus is precipitated in ethyl acetate as monomescaline citrate crystals (see Fig. 1). This technique (TEK) is specialized for catus, is relatively simple, and avoids harsh chemicals.
Thanks to everyone who contributed to this process: someblackguy, benzyme, shroombee, Metta-Morpheus, Downwardsfromzero, Kash, grollum, Mindlusion, Doubledog, Dreamer042, merkin, _Trip_, Cheelin, Highlightprotein, Loveall, and others.
Safety ⛑️
Review ethyl acetate[1] and citric acid[2] safety information. Verify solvent MSDS, plastic compatibility, and clean evaporation.
This TEK is food safe if food grade materials are used.
Following this advice does not guarantee safety. It is up to each adult individual to make their own personal decisions.
Materials🛒
Consumables👩🌾
- 300g water
- 25g Ca(OH)2 (lime)
- 100g dry cactus
- 1qt ethyl acetate (sometimes sold as "MEK substitute")
- 2.5g of citric acid (~1/2 tsp)
- Washing soda (Na2CO3) for solvent washing and reuse
Equipment🏺
- Knife, dehydrator, food processor, and coffee grinder (to harvest and make cactus powder)
- French press (optional)
- Kitchen scale (to measure ingredients)
- Coffee filters, support basket, and funnel
- Mason glass jars
- Fridge (optional)
- Milligram scale (to measure product)
- Pipette, separatory funnel, or freezer (for solvent reuse water separation)
Process Overview 👀
In short:🌵➠🟢➠🧑🏾🔬➠✨➠💖➠💚, where,
- 🌵: Grind dry cactus to a fine powder
- 🟢: Mix cactus powder to a wet alkaline paste
- 🧑🏾🔬:Pull paste with ethyl acetate
- ✨: Precipitate mescaline from ethyl acetate with citric acid
- 💖: Collect and wash monomescaline citrate crystals
- 💚: Store ethyl acetate for reuse
Detailed Process 📜
Powder 🌵
Grow[3] and harvest cactus. Store cuttings in a dark place for at least 3 months (e.g. in a paper bag or wrapped in newspaper). Data shows dark storage increases mescaline content[4]. Chop whole cacti (into for example ~1/4 inch thick slices) and dry them with (for example) a food dehydrator at low temp (~115 F).
All parts of the cactus can be used. Outer green skin yields more than the inner white core for the same dry mass[5], but mescaline is present in both parts of the plant. Outer waxy layers and spines do not yield product, they can optionally be removed but are not detrimental to the extraction.
Grind dry cactus slices to a fine powder. This can be done in two steps, first through a food processor (coarse grind) and then through a coffee grinder (fine grind).
It is very important to make a uniform cactus powder that is dry (<10% moisture) and finely ground. Clouds of fine dust fly into the air when handling a good powder, reminiscent of flour (but green). Store properly sealed to avoid moisture absorption over time (redry before extracting if needed). Old cactus powder may change to a tan color, and still works well in this process (extract may be tan instead of green).
Paste 🟢
Make milky water in a french press, quart jar, or mixing bowl by adding lime to water. Mix so there are no lumps of lime. Without giving lime time to settle, gradually add the cactus powder, stirring thoroughly to ensure the cactus is well incorporated. Give paste a fluffy, smooth texture by stirring vigorously for at least 10 minutes. In case of doubt, mix more.
This paste has the same ratios originally used in 69ron's limonene TEK [6]. However, with ethyl acetate it is very important to mix the paste well and obtain the proper texture. Some elbow grease is required, so mix with intent.
If the paste is too dry and clumpy, ethyl acetate penetration diminishes lowering yields[7]. If there is too much water the paste will emulsify with ethyl acetate lowering yields and wasting solvent (see FAQ). However, the water process window for a good paste is large, estimated at ±30ml, and with attention to detail a good paste can be made. Achieving the right paste consistency is more important than the exact amount of water added (which may vary for different cacti and pulp/skin ratios). Ideal paste consistency is reminiscent of stiff yet fluffy mashed potatoes.
Extract 🧑🏾🔬
If available, the paste should be in a french press. The french press will make it easy to decant the ethyl acetate. Optionally the paste can be in a a quart jar, but it's not as easy to decant the ethyl acetate. It is possible to have the paste in a mixing bowl, but decanting may be cumbersome.
Cover paste with a couple fingers of ethyl acetate (~200g) and stir for one minute. Do not to stir too aggressively as that may cause plant material to absorb excess solvent. A good paste becomes sandy during extraction and is very easy to handle, but other textures also work. After stirring rest for two minutes, and decant into a quart jar filtering through a coffee filter to catch unwanted plant material. If using a french press (preferred), squeeze to accelerate decanting (keeping plastic parts away from the solvent). Releasing the squeeze and re-squeezing can release more solvent. After squeezing, the compacted paste can be stirred loose for a few seconds to release a small amount of solvent and re-squeezed (repeat as needed to thoroughly complete pull).
If the paste absorbs all or most of the ethyl acetate during the first pull, that's fine. Just add more ethyl acetate, stir, rest, and decant as explained above. Do NOT add water or aggressively manipulate the paste in an attempt to force out the ethyl acetate. Some solvent absorption during the first pull is common. Even with some solvent stuck in the paste, you will still recover the vast majority of mescaline during subsequent pulls as ethyl acetate is not absorbed in subsequent pulls.
Repeat the extraction with ~125g of ethyl acetate until the extraction jar is full (5-6 total pulls). It is normal for paste to become more sticky as the pulls progress. A small yield boost can be done by optionally doing more pulls into a second jar. This second jar is also a good check on the effectiveness of the pulls in the main jar the first time this TEK is performed. Poor technique will give a larger yield boost in the second jar, <10% boost is considered good[8].
All of the extraction pulls should be completed within 45 minutes. After 45 minutes the paste can begin to congeal, making solvent penetration and recovery more difficult. There is plenty of time to lazily perform the pulls by remaining focused on the task.
Rest the combined extract for at least an hour and then inspect for droplets or particles. If present, allow extract to rest until no more debris form and remove them. With experience and confidence this step can be skipped. Conversely, inexperienced beginners are encouraged to allow extract to rest for 24 hours in a refrigerator. The extract needs to be clear, particle free, and without water droplets for the crystallization process to happen reliably (see Fig. 4).
Crystalize ✨
Drop ~2.5 grams (~1/2 teaspoon) of citric acid into extract. Rest undisturbed allowing citric acid to slowly dissolve by diffusion. Crystals of monomescaline citrate should begin to appear after a few hours. Crystals can have different shapes and can stick to the wall (sometimes looking transparent and difficult to see). After the solution clears, allow crystalization to complete (~up to 72 hours).
Fast crystalization option: Add up to 15 grams of citric acid into extract. Use a magnetic stirrer or aggressive shaking to quickly dissolve the citric acid and speed up crystallization. This produces a fast crystalization and minimizes crystals that are stuck to the wall. A stirring vortex will go from visible, to not visible as clouds form, to visible again as mescaline citrate precipitates. If shaking, clouds form and disappear within minutes, leaving product behind. Crystals will be smaller with this approach and look like a powder, but the vast majority of them will still be caught by a filter in the next step. They are denser and easier to pack in capsules, but not was pretty to look at. As with the previous option, allow crystalization to complete undisturbed (~72 hours).
Collect 💖
Swirl ethyl acetate to knock crystals loose. Crystals that cling to the wall can sometimes be dislodged by shaking or with a knife/spoon. Send solvent trough a double coffee filter to catch loose crystals. Rinse any crystals remaining on jar walls with fresh ethyl acetate and send wash through filter to also wash the crystals there and collect any new crystals that are dislodged (repeat ~2-3x until off color is mostly removed).
After the solvent washes are fully dry in the filter and jar:
- Collect crystals in the filter by simply sliding them off. Rubbing the inside of the filter against itself with the palms of the hands can help loosen the last bit of crystals. Do not introduce water into the filter since it could pick up plant matter left behind by ethyl acetate in the filter fibers (or if this is done, keep this water separate from the rest of the product in case it has plant matter).
- Collect crystals that remain stuck on the jar walls (if any) by dissolving them in warm water, evaporating water in a shallow dish, and scraping. When deciding if it is necessary to do this step keep in mind that sometimes the wall crystals form a transparent layer difficult to see.
Combine with the collected crystals to obtain the final product (Fig. 6).
Yield depends on the cactus and is usually between 0.2% to 2% with ~1.2% being common[9]. The product is monomescaline citrate salt, (MesH)H2Cit (see appendix), which is ~62% as strong as MesHCl. Approximate oral dosage recommendations for mescaline [10] roughly converted to monomescaline citrate based on molecular weight and subjective user experience:
- Threshold 100 - 200 mg
- Light 200 - 350 mg
- Common 350 - 700 mg
- Strong 700 - 1400 mg
- Heavy 1400 mg+
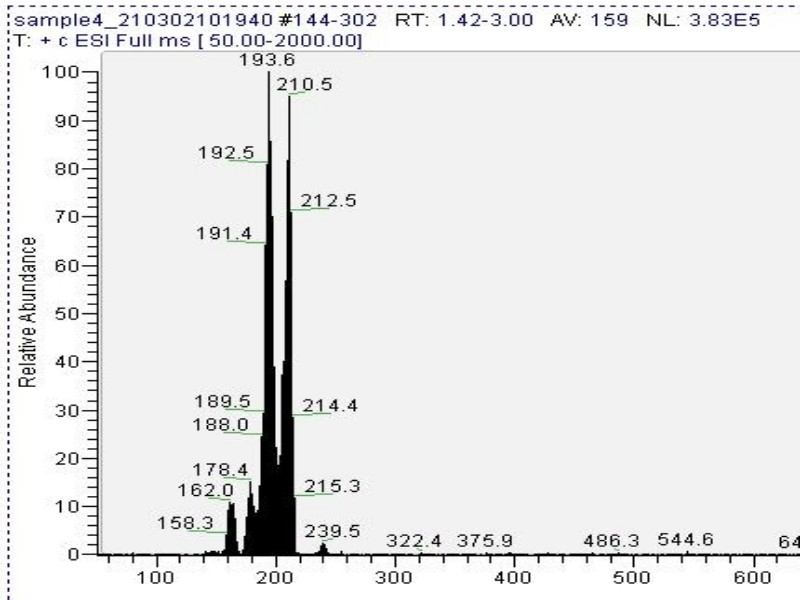
Mass spectrometry (MS) results from solaris analytical[11] indicate the product is very clean mescaline (Fig. 7) in one example where pachanoi was used. It is unknown if cacti with different starting starting alkaloid profiles would give the same result, and more data is needed to make that claim.
Reuse 💚
Reusing solvents is encouraged[12] at the DMT nexus.
Wash spent extract with sodium carbonate saturated water (35% by weight). About 1/5 of the solvent volume as saturated water is enough. Shake vigorously (emulsions do not form). CO2 bubbles may be visible during citric acid neutralization. Keep an eye on any bubbles and release any pressure buildup regularly. Neutralization can be optionally verified with pH paper. Rest washed solution until clear (up to a day or more).
Remove the water layer with a pipette or separatory funnel. Freezing the solvent and filtering the ice slush that forms with a metal strainer is also an option. Finally, decant off any excess solid sodium carbonate/citrate.
Frequently Asked Questions ❓
Q: Does increasing the basing time increase the yield?
A: No. Shroombee has tested 15 minute, 24 hour, and 72 hour basing times and there was no difference in yield. Other process variables were 8 minutes incorporating milky water with cactus, 6x3 minute pulls, and 15 mg/gram citric acid added with the fast crystallization method. Loveall has confirmed in his experiments that 10 minute and 24 hour basing times produce the same yield. So we assume that any basing time from 10 minutes through 72 hours will produce the same yield. See a detailed explanation in this post.[13]
Q: I got a en emulsion while pulling, what do I do?
A:If the paste and solvent form an emulsion, it is likely that the paste was too watery. Add lime and dry magnesium sulfate until the paste becomes chunky again and solvent is released. Keep the lime to magnesium sulfate ratio above 1 to ensure paste remains alkaline[14]. Next time, use less water to make the paste.
Q: What’s the difference between the two crystalization methods?
A: In general, adding more citric acid and aggressively stirring or shaking will:
- Force crystals to form faster
- Form smaller and denser crystals less likely to stick to the jar walls
- Cause a negligible amount of tiny crystals to drop through the coffee filter.
Q: What is the upper limit of citric acid that can be added to the extract?
A: The solubility of citric acid in ethyl acetate is over 50 mg per gram of ethyl acetate. Note that plant matter or other unwanted extraction products may affect the solubility. Stay well under 50 mg/gram to ensure no undissolved citric acid is mixed in with the mescaline citrate.
Q: After adding citric acid, I saw clouds followed by precipitation, but the precipitate reminds me of citric acid. How do I know a mescaline salt is precipitating and not citric acid?
A: Citric acid does not precipitate and stays in solution because it is well bellow its solubility limit (50mg/g) in the TEK. The white particles that form from the clouds are salts and not citric acid. A thorough swirl may be needed at the end to make sure all the added citric acid has dissolved. Once it has dissolved it will not come out of solution as citric acid.
Q: After adding citric acid, I saw clouds but didn't get crystals to precipitate, what gives?
A: First make sure the TEK instructions were followed, in particular: well mixed paste, short pull times, clean extract free of debris, citric acid is in range, etc. Before adding citric acid, allow the extract to rest. One item that has known to cause goo issues is too much water and/or presence of debris in the extract. Before adding citric acid on a next attempt, allow the extract to rest for even more time in the fridge (24+ hours). If it has excess water, drops will form on the jar walls and/or bottom that need to be decanted carefully. If nothing is precipitating, bring up the citric acid concentration up to 20mg/g and wait a few days. Check the jar walls, a transparent product may have precipitated there (e.g. this has been reported for whole bridgessi[15]). If all else fails, pulling the extract with water, evaporating, and washing with fresh ethyl acetate should leave behind a potent residue (dose will be less accurate and can be made proportional to starting cactus amount).
Q: I recovered a goo precipitate instead of crystals, what do I do?
A: There are two main options. (1) Do an A/B extraction on the goo, (2) Add the goo to cactus paste and do the TEK properly as shown by Cheelin[16] (~55% of the goo mass converted to mescaline citrate crystals).
Q: How quickly can the extraction process be done?
A: With experience, it is possible to go go from raw cactus powder to dry crystals in under an hour by skipping the debri/water check and choosing the fast crystalization method shaking the salted extract vigorously. After 5 minutes of shaking, monomescaline citrate powder can be recovered in a filter (not settling is needed). Squeezing the outsides of the filter with a paper towels or even using a hair dryer on the folded filter can accelerate the already fast ethyl acetate evaporation process.
Appendix: Development Notes 🔬
Paste 🌵
No improvements were seen with longer basing time, paste oven drying, or increasing the ionic strength with CaCl2. Microwave treatment or boiling water resulted in a small yield loss.
Paste made with sodium carbonate saturated water congeals over time and requires long solvent soaks which are darker and don't crystallize to large loose crystals (small sticky crystals were obtained).
Use of lime and boiling water causes the saponification of chlorophyll over time [17]:
Chlorophyll is soluble in Ethyl Acetate, but Chlorophyllin and Phytol are not[18]. Saponification in hot water gives an extract with less plant matter and lighter color, however yields where slightly lower with this approach.
Extract 👨🏾🔬
Tests with longer/warmer pulls resulted in darker extract, smaller crystals, solvent paste absorption, congealing of paste, and no yield benefit.
Chemically drying the extract with anhydrous CaCl2 had no benefits, while drying with MgSO4 was problematic. However, depending on the worker and techniques used, a chemical dry with CaCl2 pellets (available commercially as de-icer) could reduce water content in the solvent and possibly make crystallization easier. Washing soda (when sold as Na2CO3 in monohydrate form, or when making the anhydrous form from baking soda with an oven) may also dry the extract and be beneficial in such cases (but that is currently an assumption based on other lab techniques).
Crystalize ✨
During crystallization, excess citric acid (H3Cit) reacts with free base mescaline (Mes) to form to form the monomescaline citrate salt (MesH)H2Cit:
Monomescaline citrate salt's strength relative to mescaline HCl is 62% (ignoring any hydrate formation)[19]. By not using excess citric acid, different salt forms can be precipitated[20], but that process is more complex than the simpler excess citric acid approach.
There is a lot room for excess citric acid in solution since its solubility is 50mg/g in ethyl acetate. In extracts with crystallization issues, adding more citric acid can help force precipitation: in one example with whole cactus powder 20mg/g was used [21].
Several factors can make crystals smaller: Reusing ethyl acetate, longer/warmer pulls, higher citric acid concentration, mechanical agitation, and other potential variables. Small crystals can look like a fine powder. Potency does not seem affected by the crystallization appearance, and a powdery precipitate is not a problem unless it becomes difficult to decant/filter.
After the initial crystallization, adding more citric acid and/or moving the extract to the refrigerator does not result in any more precipitation. Moving the extract to the freezer produced ice crystals.
Other dry organic acids could work. Malic was tested but did not work as well as citric[22]. Fumaric, Tartaric, Ascorbic, Succinic, etc can be tested in future investigations.
10% sulfuric acid was tested and while some crystals formed, a separate liquid layer also appeared making the process not practical. HCl has not been tested as it may break down ethyl acetate.
Collect 💖
Washing crystals in a filter appears to wick away plant colors and is superior to decanting if the goal is white xtals.
The washed crystals in the filter can be dissolved in warm water after collecting them
(do not "pull" filter with water as that can be a source of plant matter) along with any wall crystals. This will give then final product a uniform appearance with large needles forming during slow water evaporation.
Reuse 💚
Dark extract can be cleared up with activated carbon (also called activated charcoal). Use dustless pellets (typically rinsed with water and dried before use).
While it is easier to work with a solvent that is not dark, a quantifiably benefit of using activated charcoal to decolor the used solvent is not clear. The environmental benefit of regenerating a colorless solvent is in question since ethyl acetate is easy to produce and activated charcoal requires resources to manufacture.
References 🗝️
- ↑ Ethyl acetate safety[1]
- ↑ Citric Acid Safety[2]
- ↑ Cactus growing guide[3]
- ↑ Dark storage data[4]
- ↑ Result for different cactus parts[5]
- ↑ 69ron's Limonene TEK[6]
- ↑ Minimum water paste[7]
- ↑ Second set of pulls [8]
- ↑ Cactus analysis thread[9]
- ↑ Mescaline Oral Dosage[10]
- ↑ Solaris analytical service[11]
- ↑ On reusing non polar solvent[12]
- ↑ Basing time tests results[13]
- ↑ Lime and magnesium sulfate ratio vs pH[14]
- ↑ Whole bridgessi precipitate on jar walls [15]
- ↑ Goo recovery example[16]
- ↑ Hot water saponification with lime[17]
- ↑ Phytol not present in Ethyl Acetate plant extract[18]
- ↑ Mescaline citrate vs HCl[19]
- ↑ Trimescaline citrate candidate[20]
- ↑ Ethyl acetate approach[21]
- ↑ Malic acid test[22]