Difference between revisions of "1P-LSD"
GameComplete (Talk | contribs) |
GameComplete (Talk | contribs) |
||
| Line 7: | Line 7: | ||
* Nausea, vasoconstriction (mostly when combined with other vasoconstricting substances like LSD), unusual body sensations (facial flushing, chills, goosebumps, body energy), slight increase in heart rate, difficulty focusing | * Nausea, vasoconstriction (mostly when combined with other vasoconstricting substances like LSD), unusual body sensations (facial flushing, chills, goosebumps, body energy), slight increase in heart rate, difficulty focusing | ||
| | | | ||
| − | * Mental: paranoia, fear, and panic. No other known specific physical risks at this time (but likely similar to LSD). | + | * Mental: paranoia, fear, and panic. Hallucinogen persisting perception disorder. No other known specific physical risks at this time (but likely similar to LSD). |
| | | | ||
* 1P-LSD is a substance with very little published about, with little known about its pharmacological or behavioral risks. The LD50 (median lethal dose) is not known. | * 1P-LSD is a substance with very little published about, with little known about its pharmacological or behavioral risks. The LD50 (median lethal dose) is not known. | ||
| Line 13: | Line 13: | ||
}} | }} | ||
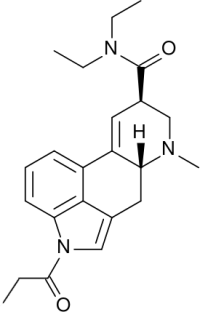
| − | [[Image:1P-LSD.png|right|thumb|200px]] | + | [[Image:1P-LSD.png|right|thumb|200px|1P-LSD]] |
== Brief overview - What is 1P-LSD? == | == Brief overview - What is 1P-LSD? == | ||
| + | * Systematic (IUPAC) name: (6aR,9R)-4-propionyl-N,N-diethyl-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide] | ||
== Chemical and physical properties == | == Chemical and physical properties == | ||
| − | + | From an old Wikipedia draft:<ref>https://en.wikipedia.org/w/index.php?title=Draft:1P-LSD&oldid=647406505</ref> | |
Sharing a strong structural similarity with [[ALD-52]], the chemical purportedly used in the 1960's Orange Sunshine 1P-LSD follows the less common path of modifying the 1- position on the LSD molecule which in the past has been avoided due to unpredictable results or lack of activity.<ref>http://chemistry.mdma.ch/hiveboard/palladium/pdf/Ergot%20-%20The%20Genus%20Claviceps%20(1999)/TF3168ch8.pdf</ref> | Sharing a strong structural similarity with [[ALD-52]], the chemical purportedly used in the 1960's Orange Sunshine 1P-LSD follows the less common path of modifying the 1- position on the LSD molecule which in the past has been avoided due to unpredictable results or lack of activity.<ref>http://chemistry.mdma.ch/hiveboard/palladium/pdf/Ergot%20-%20The%20Genus%20Claviceps%20(1999)/TF3168ch8.pdf</ref> | ||
Lots of debate is surrounding the action of 1P-LSD and is unclear exactly how the chemical is functional with an unconfirmed quote from [[David E. Nichols]] believing the chemical should be inactive while most other speculation believing it would simply follow the same metabolic path as ALD-52 and undergo conversion in vivo to LSD. | Lots of debate is surrounding the action of 1P-LSD and is unclear exactly how the chemical is functional with an unconfirmed quote from [[David E. Nichols]] believing the chemical should be inactive while most other speculation believing it would simply follow the same metabolic path as ALD-52 and undergo conversion in vivo to LSD. | ||
"I am sure that the 1-[[propionyl]] would also hydrolyze off of an [[indole]], but I don't know whether in vivo conditions would work. In a chemistry lab, you can get off an N-benzoyl, so an N-propionyl will probably come off too. But in the body? I don't know the answer to that. The compound would not be active as the N-propionyl however. Way that LSD docks into the [[5-HT2A receptor]], the indole NH hydrogen bonds to serine 5.46. With the propionyl, it won't fit into the receptor."<ref>http://www.shroomery.org/forums/showflat.php/Number/21106929#21106929</ref>. | "I am sure that the 1-[[propionyl]] would also hydrolyze off of an [[indole]], but I don't know whether in vivo conditions would work. In a chemistry lab, you can get off an N-benzoyl, so an N-propionyl will probably come off too. But in the body? I don't know the answer to that. The compound would not be active as the N-propionyl however. Way that LSD docks into the [[5-HT2A receptor]], the indole NH hydrogen bonds to serine 5.46. With the propionyl, it won't fit into the receptor."<ref>http://www.shroomery.org/forums/showflat.php/Number/21106929#21106929</ref>. | ||
| − | |||
<!--== Effects == | <!--== Effects == | ||
Revision as of 07:30, 24 February 2015
Brief overview - What is 1P-LSD?
- Systematic (IUPAC) name: (6aR,9R)-4-propionyl-N,N-diethyl-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide]
Chemical and physical properties
From an old Wikipedia draft:[1] Sharing a strong structural similarity with ALD-52, the chemical purportedly used in the 1960's Orange Sunshine 1P-LSD follows the less common path of modifying the 1- position on the LSD molecule which in the past has been avoided due to unpredictable results or lack of activity.[2] Lots of debate is surrounding the action of 1P-LSD and is unclear exactly how the chemical is functional with an unconfirmed quote from David E. Nichols believing the chemical should be inactive while most other speculation believing it would simply follow the same metabolic path as ALD-52 and undergo conversion in vivo to LSD.
"I am sure that the 1-propionyl would also hydrolyze off of an indole, but I don't know whether in vivo conditions would work. In a chemistry lab, you can get off an N-benzoyl, so an N-propionyl will probably come off too. But in the body? I don't know the answer to that. The compound would not be active as the N-propionyl however. Way that LSD docks into the 5-HT2A receptor, the indole NH hydrogen bonds to serine 5.46. With the propionyl, it won't fit into the receptor."[3].
Links
- http://www.bluelight.org/vb/threads/745848-The-Big-amp-Dandy-1P-LSD-%281-propionyl-lysergic-acid-diethylamide%29-Thread
- https://en.wikipedia.org/w/index.php?title=Draft:1P-LSD&oldid=647406505
- https://www.reddit.com/r/Drugs/comments/2s615k/new_novel_lysergamide_1plsd/