Difference between revisions of "DMT-Nexus Wiki:THH Reduction"
(→Confirmation of THH conversion) |
(→Alternative) |
||
| Line 32: | Line 32: | ||
==Alternative== | ==Alternative== | ||
Fine Magnesium ribbon can be used in the place of zinc dust. Zinc is generally considered a better option for conversion but will take longer. | Fine Magnesium ribbon can be used in the place of zinc dust. Zinc is generally considered a better option for conversion but will take longer. | ||
| + | |||
| + | ==Bases== | ||
| + | Other bases like sodium carbonate will form other salts that can be hard to separate. When using ammonia, amonnia salts formed will stay in the solution. | ||
==Confirmation of THH conversion== | ==Confirmation of THH conversion== | ||
Revision as of 12:25, 29 March 2023
Contents
THH Zinc Reduction
Introduction
Purpose of this conversion Tek is to convert harmaline to THH via zinc reduction. Using zinc to react with vinegar, hydrogen atoms form and are donated to the harmaline to form Tetrahydroharmine.
Consumables
-10g Harmaline
-400ml of Vinegar
-10g Zinc (metal) Dust or 4g of Fine Magnesium Ribbon
-10% Ammonia
-Coffee or lab filters
-Stirring utensil (strongly suggest a magnetic stirrer)
Detailed Process
-Using a glass receptacle, place 10g of Harmaline in 400ml of vinegar stir until dissolved.
-Add 10g of Zinc dust (or magnesium ribbon) leave on magnetic stirrer until the zinc/ magnesium reaction has completed and no more hydrogen bubbles are forming (this will take a number of hours). 6hours minimum for the zinc, magnesium may be shorter depending on the thickness. Note the magnesium will give a clearer hydrogen reaction.
-Using a coffee filter, filter out any left over zinc/ magnesium metal. Note lab filters will likely be required if using zinc as it is very fine (cotton balls/ funnel combination has been reported to work too).
-Add ammonia to the vinegar solution until a PH of 9.5. Tetrahydroharmine should crash out of the solution.
-Filter and dry the THH.
Alternative
Fine Magnesium ribbon can be used in the place of zinc dust. Zinc is generally considered a better option for conversion but will take longer.
Bases
Other bases like sodium carbonate will form other salts that can be hard to separate. When using ammonia, amonnia salts formed will stay in the solution.
Confirmation of THH conversion
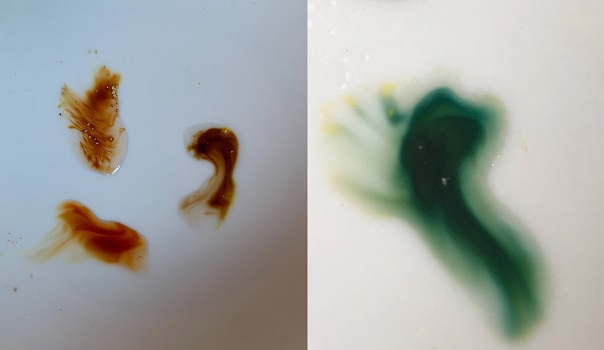
Using Marquis reagent THH should form a green color reaction. After 5-10mins the reaction will turn to brown.
Harmaline Vs THH. Photo courtesy of Merkin
Future concepts (untested)
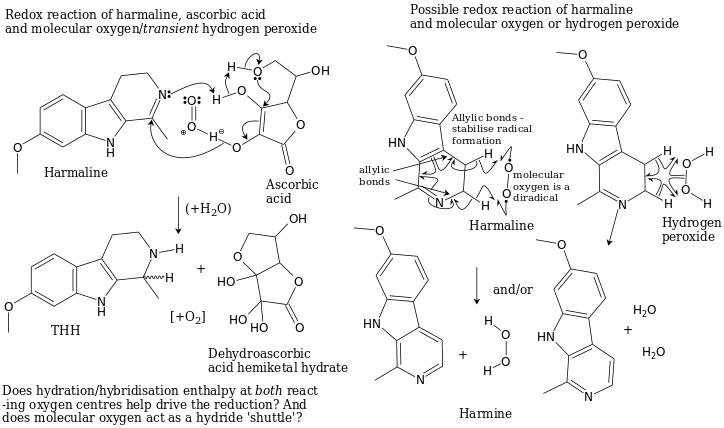
Harmaline reduction could be possible via ascorbic acid or hydrogen peroxide reactions. Graphics courtesy of Downwardsfromzero.