DMT-Nexus Wiki:THH Reduction
Contents
THH Zinc Reduction
Introduction
Purpose of this conversion Tek is to convert harmaline to THH via zinc reduction. Using zinc to react with vinegar, hydrogen atoms form and are donated to the harmaline to form Tetrahydroharmine.
Consumables
-10g Harmaline
-400ml of Vinegar
-10g Zinc (metal) Dust or 4g of Fine Magnesium Ribbon (zinc is strongly reccommended over magnesium)
-10% Ammonia
-Coffee or lab filters
-Stirring utensil (strongly suggest a magnetic stirrer)
Detailed Process
-Using a glass receptacle, place 10g of Harmaline in 400ml of vinegar stir until dissolved.
-Add 10g of Zinc dust (or magnesium ribbon) leave on magnetic stirrer until the zinc/ magnesium reaction has completed and no more hydrogen bubbles are forming (this will take a number of hours). 6hours minimum for the zinc, magnesium may be shorter depending on the thickness. Note the magnesium will give a clearer hydrogen reaction.
-Using a coffee filter, filter out any left over zinc/ magnesium metal. Note lab filters will likely be required if using zinc as it is very fine (cotton balls/ funnel combination has been reported to work too).
-Add ammonia to the vinegar solution until a PH of 9.5. Tetrahydroharmine should crash out of the solution.
-Filter and dry the THH.
Alternative
Fine Magnesium ribbon can be used in the place of zinc dust. Zinc is generally considered a better option for conversion but will take longer. This method is less tested and may not render a complete conversion.
Bases
Other bases like sodium carbonate will form other salts that can be hard to separate. When using ammonia, amonnia salts formed will stay in the solution. So stick with ammonia.
Confirmation of THH conversion
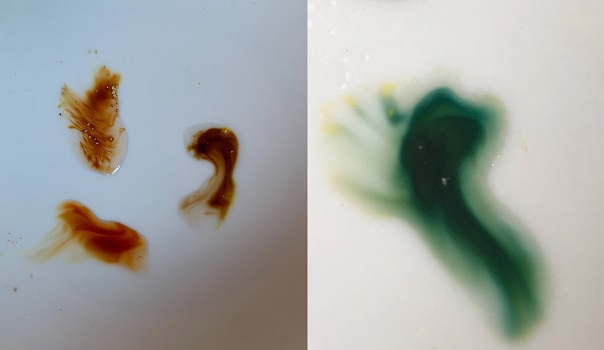
Using Marquis reagent THH should form a green color reaction. After 5-10mins the reaction will turn to brown.
Harmaline Vs THH. Photo courtesy of Merkin
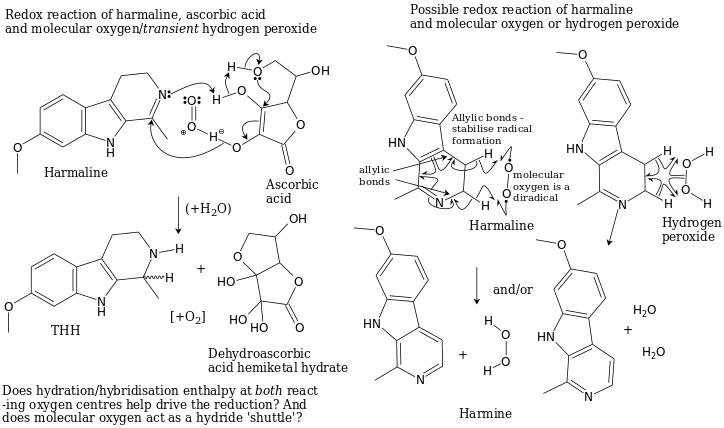
Future concepts (untested)
Harmaline reduction could be possible via ascorbic acid or hydrogen peroxide reactions. Graphics courtesy of Downwardsfromzero.