Chilled Acetone with IPA and Naphtha
Contents
Introduction
CIELO stands for Crystals In Ethyl-acetate Leisurely OTC (Over The Counter).
In this TEK, aqueous alkaline cactus paste is extracted with chilled ethyl acetate. The extract is salted with excess citric acid to force the precipitation of mescaline citrate crystals directly in the solvent.
This process was developed in a loving collaboration at the DMT nexus. Deep thanks and gratitude to everyone who contributed or provided support: someblackguy, Benzyme, shroombee, Metta-Morpheus, Downwardsfromzero, Kash, grollum, Mindlusion, Doubledog, Loveall, and others.
Safety
Review ethyl acetate's safety information[1] and check the manufacture's MSDS to verify you have pure ethyl acetate. Make sure any plastic you use is compatible with ethyl acetate and verify your ethyl acetate evaporates cleanly.
Following this advice does not guarantee safety. It is up to each adult individual to make their own decision on proceeding with this process.
Materials
- 300g water
- 25g lime
- 32 oz jar (Optional: French press)
- 100g powdered cactus (outer skin)
- ~1000g chilled ethyl acetate (freezer temperature ~ 0F )
- Large jar (e.g. 64 oz)
- Coffee filters
- Citric acid
Process
Paste
Mix water and lime in a 32oz jar (or optionally a french press) to make milk of lime. Incorporate cactus while stirring until paste is smooth. Allow paste to react for 24 hours and mix again for a few minutes.
Pull
Add ~ 300g of freezer chilled ethyl acetate to the paste, mix for 60 seconds, and decant/filter to a large jar (64 oz). If using a french press squeeze very gently to avoid pulling water. Quickly repeat 5 more times with ~150g of ethyl acetate.
Image below shows how the extract should look: clear with no droplets or particles.
Salt
Verify no particles or droplets are in the extract. If any are present, decant/filter to remove them.
Gently drop ~ 2g of citric acid into the extract. Do not stir, allow citric acid to dissolve by diffusion. During the next few hours, solution will become cloudy and crystals will form. Once solution is clear, move it to the fridge for 24h to finish crystalization.
Finish
Swirl salted extract to suspend crystals in solution and catch them in a coffee filter. Rinse jar walls with a small amount of fresh ethyl acetate to pickup any remaining crystals. Repeat this about two more times until green color is removed and all crystals are in the filter.
Dry and collect crystals. This is the final product:
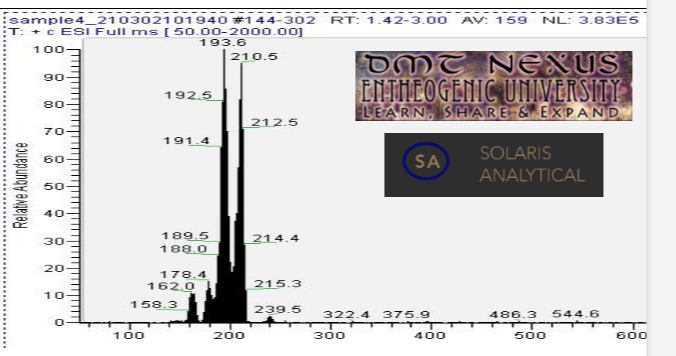
Mass spectrometry (MS) results from solaris analytical[2] indicate the product is very clean mescaline. See MS spectrum below, peak near 210.5 is mescaline. Peaks at and 193.6, 178.4, and 162.0 are believed to be mescaline with amine/methyl/methoxy groups cleaved to generate the lower mass mescaline spectrum in multiples of ~16 au (16.9, 32.1, and 48.5 respectively). The small peak at 239.5 is not attributed to mescaline.
Appendix: Lab Notes
Chilled ethyl acetate was found to make the process robust experimentally, presumably by minimizing plant material in the extract while remaining efficient for pulling mescaline.
It is also possible to obtain good results with quick room temperature ethyl acetate (3 minute pulls), but the crystals may be smaller, stick to the walls, and have a tan color.
Long room temperature pulls made the paste congeal and resulted in lower yield. Heating during the pull was tested and did not improve yields.
Adding NaCl to the paste before pulling was also tested, but no improvements were noted.
It is possible to chemically dry the extract with a drying agent such as anhydrous MgSO4 before salting. However, no clear yield benefit was observed by performing this step. In one example, drying the extract with CaCl2 resulted in a more difficult crystalization and a 40% yield loss. Apparently, any water content in ethyl acetate directly from the pulls is naturally in a good range experimentally.
Droplets or debris not decanted/filtered before salting may result in poor crystalization for any pull condition. If issues are encountered while salting, adding more citric acid and shaking may help.
During salting, every 10mg of citric acid (H3Cit) reacts with enough free base mescaline (Mes) to form to 43mg of mescaline citrate (or slightly more if a hydrate form is precipitating):
250mg of citric acid are enough to convert mescaline from free base to salt for a typical cactus (100g with up to 1% yield). However, and outlier like the legendary Ogun would need ~1100mg of citric acid for a 4.7% yield for 100g of dry cactus. The excess amount used in this the TEK guarantees salting of the most potent of cacti.
Excess acid induces crystalization. This is a simple but very important lab observation, compatible with Le Chatelier's principle. There is room for excess citric acid in solution since 50mg can dissolve per gram of ethyl acetate (50mg/g). Water in the solvent from the pulls may increase this solubility further. The vast majority of excess acid is poured off after salting, and remaining traces are removed when washing the crystals. No new or additional crashes are observed over acidifying after the first crash. The TEK recommends 2mg/g since this was found to be enough to crash mescaline for most users, but others may need more citric acid.
The crystalization could depend on the amount of plant material in the extract (which can be variable between plant sources and in the solvents temperature). If working with room temperature solvent, Sometimes it can take longer for crystals to form and a few days are needed. Other times crystalization can be very quick when using a magnetic stirrer. It has also been reported that with warm solvent 5mg/g of citric acid is enough when working with outer cactus skin (but not when working with the whole plant). While there is some variability between experiments, given enough citric acid and time, mescaline citrate crystals eventually form from a clean extract in most situations, even if room temperature ethyl acetate is used instead of the recommended chilled ethyl acetate.
Other solid organic acids could work. Fumaric, Malic, Tartaric, Ascorbic, Succinic, etc can be tested. Sulfuric acid HCl could be investigated (and crystals have been observed with sulfuric), but may interact with ethyl acetate and break it down, an issue not expected with the milder organic acids.
It is possible that some of the assumptions and conclusions in these lab notes are incorrect or incomplete. The process was tested in several ways, but the search was not exhaustive[3]. There could be ways to improve this process.