Difference between revisions of "Chilled Acetone with IPA and Naphtha"
(→3 Dry) |
(→De-polymerization💔) |
||
| (1,098 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
| − | + | =Introduction 🙏= | |
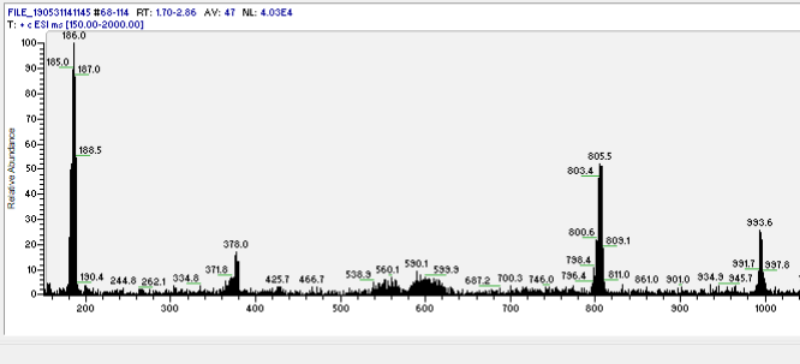
| + | Pure DMT free base can form white crystals, yellow powder, and orange to red wax/goo. This wide range of appearance could be due to self aggregation because of indole ring pi bond stacking <ref>Polymer MS evidence[https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=88183]</ref> (see Fig. 1). | ||
| − | + | This technique (TEK) focuses on maximizing white crystalline DMT by minimizing self aggregation during extraction. | |
| − | |||
| − | + | Thanks to benzyme for showing MS evidence of DMT weakly bonding to itself, and to Jees, downwardsfromzero, IridiumAndLace, and Loveall for their contributions to this process in the forum<ref>Minimum Polymer[https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=97103]</ref>. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | '' | + | [[File:Dmt copy 800x364.png| center]] |
| + | <center>''Fig. 1: Mass spectrum of DMT goo (from benzyme). Peaks in multiples of 188m/z unmask the nature of DMT goo as DMT-DMT bonding aggregation (possibly through indole ring pi stacking).</center> | ||
| − | [[ | + | = Safety ⛑️= |
| + | Review NaOH<ref>NaOH safety[https://www.cdc.gov/niosh/topics/sodium-hydroxide/default.html]</ref> and naphtha <ref>Naphtha safety[https://www.cdc.gov/niosh/npg/npgd0664.html]</ref> safety information. Verify solvent MSDS purity, plastic compatibility, and clean evaporation. | ||
| − | |||
| − | + | Never have solvents near an open flame. | |
| − | + | Following this advice does not guarantee safety. It is up to each adult individual to make their own decision. | |
| − | == | + | =Materials🛒= |
| + | ==Consumables👩🌾== | ||
| + | * 800ml water | ||
| + | * 100g of mimosa hostilis root bark | ||
| + | * 10g ascorbic acid (Vitamin C) | ||
| + | * 50g KCl | ||
| + | * 250ml of '''light''' naphtha/hydrocarbons† | ||
| + | * 25g of NaOH | ||
| − | |||
| − | + | †''It is very important to use a source of light hydrocarbons (~8 carbon chains or lower). The smaller organic molecules used in lighter fluids seem to reduce DMT aggregation. Naptha used in paint thinning applications tends to be too heavy (10+ carbon chains). Ronsonol is a good lighter fluid choice available over the counter. Avoid products with anti rust or dyes (e.g. Coleman camping fuel).'' | |
| + | ==Equipment🏺== | ||
| + | * Stovetop | ||
| + | * Pot with lid | ||
| + | * Quart jars | ||
| + | * Scale | ||
| + | * Pipette | ||
| + | * Shallow pyrex baking dish | ||
| + | * Freezer | ||
| + | * Fan | ||
| + | * Scraping tool | ||
| − | + | = Process Overview 👀 = | |
| + | *Cell lysing❄️: In a small pot, freeze/thaw powdered bark and water three times | ||
| + | *De-polymerize💔: Add citric acid together with KCl and brew at 150F for an hour and cool | ||
| + | *Pull👩🔬: Add light hydrocarbon solvent, basify with NaOH, shake and pull warm solvent at ~120F. Repeat 5x | ||
| + | *Collect✨: Freeze precipate solvent<sup>†</sup>, decant, dry, and scrape | ||
| − | + | ''<sup>†</sup>Evaporation is skipped and max yield is achieved on reused solvent. | |
| − | + | = Detailed Process 📜= | |
| + | == Cell Lysing ❄️== | ||
| + | Freeze/thaw bark mixed with 800ml of water in a pot with a lid. Repeat twice for a total of 3 times. Process can be sped up defrosting over low heat. | ||
| + | ==De-polymerization💔== | ||
| + | Stir in ascorbic acid and KCl. Heat gently to 150F. Cover pot with lid and keep it at this temperature (e.g. using very low heat) for one hour. | ||
| − | |||
| + | Ascorbic acid and plant enzymes degrade at high temperatures, especially above 150F-175F. K+ ions are good at disturbing DMT pi bond aggregation in water and superior to Na+ ions. | ||
| − | Pull | + | == Pull 👩🔬== |
| + | Transfer treated liquid and bark to a mason quart jar (or another suitable container). Add water if needed so quart jar is close to being full. Shake in ~65ml of light naphtha. Add lye and shake vigorously for a few minutes. Solution will warm up slightly as lye dissolves and will quickly go from red, to milky, to dark red. | ||
| − | + | Rest jar in a warm water bath until naphtha layer separates (~10 minutes, see Fig. 3). If separation is not complete after 30 minutes, mix in another 5g of lye and try again. | |
| − | + | Move naphtha into a pint jar with a pipette It is ok if a few drops of watery extract or bark particles come through (they will be decanted in the next section). | |
| − | |||
| − | + | Add another ~65ml of naphtha to the quart jar. Shake for a few minutes, rest in a warm water bath until layers separate, and pipette naphtha into the pint jar. Perform this step two more times (total of 4 pulls, including the first one). | |
| − | |||
| − | + | Ideally, all four pulls are done within an hour while the quart jar is slightly warm from the lye dissolving in water. | |
| − | + | [[File:IMG 20211020 090639578 copy 600x1122 copy 427x800.jpg|center]] | |
| + | <center>''Fig. 3: Settled naphtha pull ready to be pipetted.</center> | ||
| + | == Crystalize ✨== | ||
| + | Carefully decant naphtha pulls to a new fresh pint jar. Do not allow any watery extract or particles to come through. | ||
| − | |||
| + | Place naphtha in freezer to precipitate crystals<sup>†</sup>. Rest in freezer until cloudiness clears (at least 24 hours). | ||
| − | |||
| + | Decant naphtha off crystals, and immediately dry with the help of a fan. Once dry, dissolve xtals in a minimal amount of boiling fresh naphtha (~25ml) for 15 minutes, pout into a shallow baking dish, evaporate slowly (no fan), and scrape. This is the final product. Yields are typically 1 to 3%. | ||
| − | |||
| + | ''<sup>†</sup>If new naphtha was used, one option is to evaporate the solvent until slightly cloudy with the help of a fan in a well ventilated area. A better option is to skip the solvent evaporation. Yield will be lower by ~500mg if using new naphtha, but it will be available for reuse as a one-time "investment" for the next extraction. Subsequently, used naphtha does not need to be evaporated before freezing to get the full yield since it already comes preloaded with a DMT concentration that is saturated at the freezer's temperature.'' | ||
| − | + | == Reclaim Solvent 💚== | |
| + | Reusing solvents is encouraged<ref>On reusing non polar solvent[https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=31398]</ref> at the DMT nexus. | ||
| − | |||
| − | + | Simply reuse freeze precipitated naphtha as-is. Re-used naphtha is saturated with DMT at freezer the temperature (~2mg/ml) and pre-freezer evaporation is not needed. Easy 😊 | |
| + | = Frequently Asked Questions ❓ = | ||
| + | '''Q: That's a lot of hypothesis you got down in the appendix. Have any experimental evidence consistent with them?''' | ||
| − | + | A: Yes. Benzyme's MS, together with polymerization and de-polymerization experiments. As far as we know experiments are consistent with the hypotheses listed. The community is welcome to update this Wiki entry as more evidence arises, especially if any of the hypotheses are disproved (thank you). | |
| − | '' | + | '''Q: What's so special about Vitamin C?''' |
| − | == | + | A: See the development notes in appendix below. |
| + | |||
| + | |||
| + | '''Q: Why are there only 3 pulls without a warm water bath or salting out ionic strength? Usually ~5 warm (40-50C) + high ionic strength pulls (~6% NaCl) are needed.''' | ||
| + | |||
| + | A: DMT monomer is highly soluble in naphtha and has an excellent partition coefficient. By converting natural DMT to this form, and keeping alkaline conditions gentle to avoid polymerization, the pulls are simpler and very efficient. No added heat or ionic strength is necessary. | ||
| + | |||
| + | |||
| + | '''Q: What is the difference between DMT polymers, oligomers, aggregates, and aromatic pi-pi stacking?''' | ||
| + | |||
| + | A: None, all names are equivalent and refer to the same thing: weakly bonded groups of DMT molecules that form goo instead of crystals. | ||
| + | |||
| + | = Appendix: Development Notes 🔬= | ||
| + | == Hypotheses 🤔== | ||
| + | This TEK hypothesizes that: | ||
| + | |||
| + | |||
| + | *Not all of the DMT is in the plant in monomer form, some of it is in macro-molecule form (also called polymer, oligomer, or goo) | ||
| + | *In addition to natural DMT polymer, even more polymer can form during the basing step under high alkaline, high ionic strength, and high DMT concentration conditions | ||
| + | *Once natural DMT polymer is broken down, gentle alkaline conditions keep it from forming again | ||
| + | *Goo can also form in the solvent. Using lighter naphtha (shorter carbon chains) minimizes DMT goo formation. | ||
| + | *DMT monomer properties compared to DMT polymer: | ||
| + | **Easier to dissolve in naphtha (better partition coefficient) | ||
| + | **Barely clouds during naphtha evaporation | ||
| + | **Slowly crashes during freeze precipitation as white crystals. In contrast, DMT polymer precipitates sooner as yellow/orange/red semisolid goo | ||
| + | **Easier to handle and dose precisely | ||
| + | **Low and consistent vaporization temperature, ideal for newer electronic vaporization devices with precisely tuned temperature settings | ||
| + | **Visibly unique upon crystalization, eliminating questions around plant oil contaminants | ||
| + | **May be easier to complex with HPBCD for sublingual administration | ||
| + | **It is unknown if it has better bioavailability for oral or rectal administration. In principle, stomach acid should be able to break down DMT polymer, so perhaps there is no difference for oral administration | ||
| + | **There is no expected benefit for torch vaporization by an experienced user since the strong heat produced manually can easily vaporize everything. However, the process window between vaporizing and burning the DMT is larger for the monomer which may benefit the inexperienced user | ||
| + | |||
| + | ==Strategy ♟️== | ||
| + | The strategy of this minimum polymer TEK is to break down both natural DMT aggregates during the acid step and minimize DMT aggregation during the basing and pulling steps. | ||
| + | |||
| + | |||
| + | Aggressive alkaline concentration conditions are avoided. While these type of processes can break down plant material, their downside is that they don't break down natural DMT aggregates and can even increase the degree of polymerization. | ||
| + | |||
| + | |||
| + | Fortunately, DMT aggregates can break down in acidic conditions. Therefore, to simultaneously break down DMT aggregates and plant material, a long acidic pressure cooking step is used (described before by for example Northener). Vitamin C is used to complete de-aggregation due to its good experimental performance and some literature references referring to it's ability to disrupt pi-pi bonds<ref>Uric acid de-aggregation by vitamin C[https://pubs.rsc.org/en/content/articlelanding/2021/cp/d1cp01504d/unauth]</ref>, but other acids could also work. Subsequently, relatively gentle ionic strength (no added salt), gentle alkaline pH (no excess lye beyond emulsion breakdown), and low DMT concentration (<0.5%) conditions are used to minimize any DMT re-polymerization. Naphtha is introduced before basing to minimize the time bulk DMT spends in alkaline water. | ||
| + | |||
| + | == Vitamin C 🍊== | ||
| + | Experimentally, Vitamin C produced better results compared to acetic and citric acids. Vitamin C is biologically active as a mild antioxidant and reducing agent and can pass through cell membranes. | ||
| + | |||
| + | |||
| + | Vitamin C begins to degrade at 158F. The activity of vitamin C decreases with temperature, so it is added when the extract is still hot yet below this degradation temperature. | ||
| + | |||
| + | |||
| + | A possible specific mechanism of action is that as a strong electron donor, vitamin C disrupts parallel displaced aromatic ring pi-bond stacking conformations<ref>Pi-bond aromatic stacking[https://en.m.wikipedia.org/wiki/Pi-Stacking_(chemistry)]</ref><ref>Tryptophan parallel displaced stacking[https://www.jbc.org/article/S0021-9258(18)80815-8/fulltext]</ref>. | ||
| + | |||
| + | |||
| + | Other acids may also work, and the kitchen alchemist is encouraged to report on any new experimental results (both positive and negative). | ||
| + | |||
| + | == Cloudiness 🌫️== | ||
| + | DMT monomer does not readily form clouds in naphtha compared to other extractions that do not minimize polymer. In this TEK clouds form later in the freezer or evaporation process and are not as opaque. Late cloud formation is a good sign and not a cause for concern. Monomer crystals take longer to grow in the freezer, so give them extra time. | ||
| + | |||
| + | = References 🗝️= | ||
<references/> | <references/> | ||
Latest revision as of 14:22, 11 July 2022
Contents
Introduction 🙏
Pure DMT free base can form white crystals, yellow powder, and orange to red wax/goo. This wide range of appearance could be due to self aggregation because of indole ring pi bond stacking [1] (see Fig. 1).
This technique (TEK) focuses on maximizing white crystalline DMT by minimizing self aggregation during extraction.
Thanks to benzyme for showing MS evidence of DMT weakly bonding to itself, and to Jees, downwardsfromzero, IridiumAndLace, and Loveall for their contributions to this process in the forum[2].
Safety ⛑️
Review NaOH[3] and naphtha [4] safety information. Verify solvent MSDS purity, plastic compatibility, and clean evaporation.
Never have solvents near an open flame.
Following this advice does not guarantee safety. It is up to each adult individual to make their own decision.
Materials🛒
Consumables👩🌾
- 800ml water
- 100g of mimosa hostilis root bark
- 10g ascorbic acid (Vitamin C)
- 50g KCl
- 250ml of light naphtha/hydrocarbons†
- 25g of NaOH
†It is very important to use a source of light hydrocarbons (~8 carbon chains or lower). The smaller organic molecules used in lighter fluids seem to reduce DMT aggregation. Naptha used in paint thinning applications tends to be too heavy (10+ carbon chains). Ronsonol is a good lighter fluid choice available over the counter. Avoid products with anti rust or dyes (e.g. Coleman camping fuel).
Equipment🏺
- Stovetop
- Pot with lid
- Quart jars
- Scale
- Pipette
- Shallow pyrex baking dish
- Freezer
- Fan
- Scraping tool
Process Overview 👀
- Cell lysing❄️: In a small pot, freeze/thaw powdered bark and water three times
- De-polymerize💔: Add citric acid together with KCl and brew at 150F for an hour and cool
- Pull👩🔬: Add light hydrocarbon solvent, basify with NaOH, shake and pull warm solvent at ~120F. Repeat 5x
- Collect✨: Freeze precipate solvent†, decant, dry, and scrape
†Evaporation is skipped and max yield is achieved on reused solvent.
Detailed Process 📜
Cell Lysing ❄️
Freeze/thaw bark mixed with 800ml of water in a pot with a lid. Repeat twice for a total of 3 times. Process can be sped up defrosting over low heat.
De-polymerization💔
Stir in ascorbic acid and KCl. Heat gently to 150F. Cover pot with lid and keep it at this temperature (e.g. using very low heat) for one hour.
Ascorbic acid and plant enzymes degrade at high temperatures, especially above 150F-175F. K+ ions are good at disturbing DMT pi bond aggregation in water and superior to Na+ ions.
Pull 👩🔬
Transfer treated liquid and bark to a mason quart jar (or another suitable container). Add water if needed so quart jar is close to being full. Shake in ~65ml of light naphtha. Add lye and shake vigorously for a few minutes. Solution will warm up slightly as lye dissolves and will quickly go from red, to milky, to dark red.
Rest jar in a warm water bath until naphtha layer separates (~10 minutes, see Fig. 3). If separation is not complete after 30 minutes, mix in another 5g of lye and try again.
Move naphtha into a pint jar with a pipette It is ok if a few drops of watery extract or bark particles come through (they will be decanted in the next section).
Add another ~65ml of naphtha to the quart jar. Shake for a few minutes, rest in a warm water bath until layers separate, and pipette naphtha into the pint jar. Perform this step two more times (total of 4 pulls, including the first one).
Ideally, all four pulls are done within an hour while the quart jar is slightly warm from the lye dissolving in water.
Crystalize ✨
Carefully decant naphtha pulls to a new fresh pint jar. Do not allow any watery extract or particles to come through.
Place naphtha in freezer to precipitate crystals†. Rest in freezer until cloudiness clears (at least 24 hours).
Decant naphtha off crystals, and immediately dry with the help of a fan. Once dry, dissolve xtals in a minimal amount of boiling fresh naphtha (~25ml) for 15 minutes, pout into a shallow baking dish, evaporate slowly (no fan), and scrape. This is the final product. Yields are typically 1 to 3%.
†If new naphtha was used, one option is to evaporate the solvent until slightly cloudy with the help of a fan in a well ventilated area. A better option is to skip the solvent evaporation. Yield will be lower by ~500mg if using new naphtha, but it will be available for reuse as a one-time "investment" for the next extraction. Subsequently, used naphtha does not need to be evaporated before freezing to get the full yield since it already comes preloaded with a DMT concentration that is saturated at the freezer's temperature.
Reclaim Solvent 💚
Reusing solvents is encouraged[5] at the DMT nexus.
Simply reuse freeze precipitated naphtha as-is. Re-used naphtha is saturated with DMT at freezer the temperature (~2mg/ml) and pre-freezer evaporation is not needed. Easy 😊
Frequently Asked Questions ❓
Q: That's a lot of hypothesis you got down in the appendix. Have any experimental evidence consistent with them?
A: Yes. Benzyme's MS, together with polymerization and de-polymerization experiments. As far as we know experiments are consistent with the hypotheses listed. The community is welcome to update this Wiki entry as more evidence arises, especially if any of the hypotheses are disproved (thank you).
Q: What's so special about Vitamin C?
A: See the development notes in appendix below.
Q: Why are there only 3 pulls without a warm water bath or salting out ionic strength? Usually ~5 warm (40-50C) + high ionic strength pulls (~6% NaCl) are needed.
A: DMT monomer is highly soluble in naphtha and has an excellent partition coefficient. By converting natural DMT to this form, and keeping alkaline conditions gentle to avoid polymerization, the pulls are simpler and very efficient. No added heat or ionic strength is necessary.
Q: What is the difference between DMT polymers, oligomers, aggregates, and aromatic pi-pi stacking?
A: None, all names are equivalent and refer to the same thing: weakly bonded groups of DMT molecules that form goo instead of crystals.
Appendix: Development Notes 🔬
Hypotheses 🤔
This TEK hypothesizes that:
- Not all of the DMT is in the plant in monomer form, some of it is in macro-molecule form (also called polymer, oligomer, or goo)
- In addition to natural DMT polymer, even more polymer can form during the basing step under high alkaline, high ionic strength, and high DMT concentration conditions
- Once natural DMT polymer is broken down, gentle alkaline conditions keep it from forming again
- Goo can also form in the solvent. Using lighter naphtha (shorter carbon chains) minimizes DMT goo formation.
- DMT monomer properties compared to DMT polymer:
- Easier to dissolve in naphtha (better partition coefficient)
- Barely clouds during naphtha evaporation
- Slowly crashes during freeze precipitation as white crystals. In contrast, DMT polymer precipitates sooner as yellow/orange/red semisolid goo
- Easier to handle and dose precisely
- Low and consistent vaporization temperature, ideal for newer electronic vaporization devices with precisely tuned temperature settings
- Visibly unique upon crystalization, eliminating questions around plant oil contaminants
- May be easier to complex with HPBCD for sublingual administration
- It is unknown if it has better bioavailability for oral or rectal administration. In principle, stomach acid should be able to break down DMT polymer, so perhaps there is no difference for oral administration
- There is no expected benefit for torch vaporization by an experienced user since the strong heat produced manually can easily vaporize everything. However, the process window between vaporizing and burning the DMT is larger for the monomer which may benefit the inexperienced user
Strategy ♟️
The strategy of this minimum polymer TEK is to break down both natural DMT aggregates during the acid step and minimize DMT aggregation during the basing and pulling steps.
Aggressive alkaline concentration conditions are avoided. While these type of processes can break down plant material, their downside is that they don't break down natural DMT aggregates and can even increase the degree of polymerization.
Fortunately, DMT aggregates can break down in acidic conditions. Therefore, to simultaneously break down DMT aggregates and plant material, a long acidic pressure cooking step is used (described before by for example Northener). Vitamin C is used to complete de-aggregation due to its good experimental performance and some literature references referring to it's ability to disrupt pi-pi bonds[6], but other acids could also work. Subsequently, relatively gentle ionic strength (no added salt), gentle alkaline pH (no excess lye beyond emulsion breakdown), and low DMT concentration (<0.5%) conditions are used to minimize any DMT re-polymerization. Naphtha is introduced before basing to minimize the time bulk DMT spends in alkaline water.
Vitamin C 🍊
Experimentally, Vitamin C produced better results compared to acetic and citric acids. Vitamin C is biologically active as a mild antioxidant and reducing agent and can pass through cell membranes.
Vitamin C begins to degrade at 158F. The activity of vitamin C decreases with temperature, so it is added when the extract is still hot yet below this degradation temperature.
A possible specific mechanism of action is that as a strong electron donor, vitamin C disrupts parallel displaced aromatic ring pi-bond stacking conformations[7][8].
Other acids may also work, and the kitchen alchemist is encouraged to report on any new experimental results (both positive and negative).
Cloudiness 🌫️
DMT monomer does not readily form clouds in naphtha compared to other extractions that do not minimize polymer. In this TEK clouds form later in the freezer or evaporation process and are not as opaque. Late cloud formation is a good sign and not a cause for concern. Monomer crystals take longer to grow in the freezer, so give them extra time.