Difference between revisions of "Chilled Acetone with IPA and Naphtha"
(→Introduction 🙏) |
(→Safety ⛑️) |
||
| Line 9: | Line 9: | ||
Thanks to everyone who contributed in this collaborative effort: someblackguy, Benzyme, shroombee, Metta-Morpheus, Downwardsfromzero, Kash, grollum, Mindlusion, Doubledog, Dreamer042, Loveall, and others. | Thanks to everyone who contributed in this collaborative effort: someblackguy, Benzyme, shroombee, Metta-Morpheus, Downwardsfromzero, Kash, grollum, Mindlusion, Doubledog, Dreamer042, Loveall, and others. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
= Materials 🛒= | = Materials 🛒= | ||
Revision as of 18:52, 8 June 2021
Contents
Introduction 🙏
CIELO stands for Crystals In Ethyl-acetate Leisurely Over-the-counter.
In this technique (TEK), alkaline cactus paste (Fig. 2) is extracted with chilled ethyl acetate (Fig. 3). Mescaline citrate is precipitated by citric acid (Fig. 4-5) and collected (Fig. 6).
Thanks to everyone who contributed in this collaborative effort: someblackguy, Benzyme, shroombee, Metta-Morpheus, Downwardsfromzero, Kash, grollum, Mindlusion, Doubledog, Dreamer042, Loveall, and others.
Materials 🛒
- Mescaline Extraction:
- Quart jar or french press
- 300g water
- 25g lime
- 100g dry cactus powder (outer skin or whole cactus)
- ~1200g ethyl acetate (sometimes found as "MEK substitute")
- Paper coffee filter, support basket, and funnel
- Large jar (~64 oz)
- 5g of citric acid if using outer cactus skin or 20g if using whole cactus
- Shallow baking dish, lid, and aluminum foil
- Razor blade
- Solvent Reclaim:
- Washing soda
- Water
- pH paper strips (optional)
- Separatory funnel or glass turkey baster
- Coffee filter/filter basket/funnel
Process 📜
Make Alkaline Paste 🌵
Mix water and lime in a quart jar or french press. Incorporate cactus while stirring and continue stirring for at least 10 minutes to a smooth paste.
Pull Alkaline Paste 👩🔬
Add ~ 300g of freezer chilled ethyl acetate (~0 F) to the paste, mix thoroughly for for 60 seconds, and decant/filter to a large jar (~64 oz). If using a french press squeeze very gently or not at all to avoid pulling water. Immediately pull 5 more times with ~150g of ethyl acetate stirring thoroughly for 60 seconds each time.
Inspect extract for droplets or particles. If present, decant/filter to remove them.
Extract needs to be clean (see Fig. 3).
Crystalize Extract 🧪
Let extract reach room temperature and pour it into a shallow baking dish. Gently drop in 5g of citric acid if using outer skin powder or 20g if using whole cactus powder. Do not stir, simply allow citric acid to diffuse into solution forming clouds (Fig. 4).
Cover with a lid protected by a layer of aluminum foil if needed (most shallow baking dish lids are not resistant to ethyl acetate). After a few hours clouds crash as mescaline citrate crystals (Fig. 5). Allow enough time to complete crystallization (~24h is typically sufficient but can vary).
Collect Crystals ✨
Decant and rinse crystals with a small amount of fresh ethyl acetate twice (or until off color is mostly removed). Allow crystals to dry and collect them from the shallow baking dish with a razor blade to obtain the final product (Fig. 6).
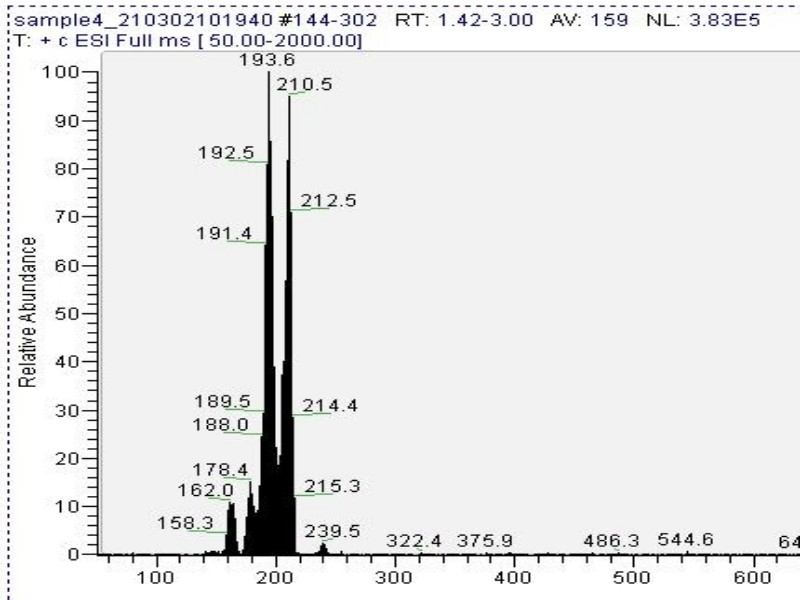
Mass spectrometry (MS) results from solaris analytical[1] indicate the product is very clean mescaline (Fig. 7). Yield is dependent on cactus material and usually between 0.2% to 2% [2] with ~1% being common.
Reclaim Solvent 💚
Reusing solvents is encouraged[3] at the DMT nexus.
Wash spent extract with sodium carbonate saturated water shaking vigorously (emulsions do not form). Citric acid removal can be optionally verified with a strip of pH paper. Separate water layer. Freeze and filter out ice crystals. Store for reuse.
Appendix: Development Notes 🔬
Paste 🌵
No improvements were seen with longer basing time, microwaving, partially of completely drying (with heat or drying agent), or adding NaCl to increase the ionic strength.
Pull 👨🏾🔬
It is also possible to obtain good results with 3 minute room temperature pulls (30s of stirring and 150s of rest), but the results may not be as consistent compared to chilled ethyl acetate (60s of stirring). This is thought to be because of increased plant contaminants in the warmer pull at room temperature making crystallization more difficult.
Long room temperature pulls made the paste congeal and resulted in lower yield. Heating during the pull did not improve yields. Generally, longer times or higher temperatures make the crystalization more difficult without yield improvements and a persistent tan color can contaminate the product.
Extract 🧪
Chemically drying the extract with a drying agent such as anhydrous MgSO4 before salting had no yield benefit. In one example, drying the extract with CaCl2 resulted in a more difficult crystalization and a 40% yield loss. In general, any water content in ethyl acetate directly from the pulls is already in a good range experimentally.
Droplets or debris not decanted/filtered before adding citric acid can result in crystalization issues. It is very important to verify the extract is clean as mentioned in the main TEK.
Crystalization ✨
During crystallization, 233mg of citric acid (H3Cit) react with free base mescaline (Mes) to form to 1g of mescaline citrate (or slightly more if a hydrate is precipitating):
The TEK calls for more citric acid than would be needed for titration because excess citric acid shifts the precipitation reaction to the right (Le Chatelier's principle), helping overcome water and plant material. There is a lot room for excess citric acid in solution since its solubility is 50mg/g in ethyl acetate. The TEK recommends 5mg/g for outer skin cactus powder and 20mg/g for whole cactus powder based on early experiments[4], but since cacti and pull techniques can vary, users may find other values work better for their specific situation.
Mescaline citrate grows as needles in ethyl acetate (Fig. 4) that can be up to several mm long. Reused ethyl acetate, warmer pulls, longer pulls, higher citric acid concentration, agitation, and colder temperatures tend to make the needles smaller. Very small needles can look like a powder. Potency does not seem affected by the crystallization appearance, and a powdery precipitate is not an problem unless it becomes difficult to decant/filter.
After the main TEK's room temperature crystallization, adding more citric acid and/or moving the extract to the refrigerator did not result in any more precipitation. Moving the extract to the freezer produced only ice crystals.
Other dry organic acids could work. Fumaric, Malic, Tartaric, Ascorbic, Succinic, etc can be tested in future investigations.
Disclaimer 🤷♂️
It is possible that some of the assumptions and conclusions in these lab notes are incorrect or incomplete. The process was tested in several ways, but the search was not exhaustive. There could be ways to improve this process.