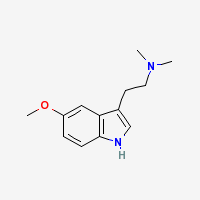
5-MeO-DMT
| Note: | This page has been transcluded to The Nexian DMT Handbook under the 5-MeO-DMT section or other locations within or without the handbook. Please markup in consideration of this. The top section header is to remain in place as a reference for subsequent section headers and to allow easy editing directly from the handbook. |
Contents
- 1 Brief overview - What is 5-MeO-DMT?
- 2 Chemical and physical properties
- 3 Effects
- 4 Pharmacology, toxicity and general safety
- 5 Plants containing 5-MeO-DMT
- 6 Extraction teks
- 7 Dosages and consumption methods
- 8 History of usage
- 9 Analysis of 5-MeO-DMT
- 10 Scientific publications
- 11 Other links of interest
Brief overview - What is 5-MeO-DMT?
5-MeO-DMT is a potent naturally occuring psychedelic alkaloid
Chemical and physical properties
For solubility, melting point, etc, check the 5-MeO-DMT Chemical and Physical Properties WIKI
Effects
Excessively fast onset - 10-30 seconds.
Lower doses (2-6 mgs smoked) - Crushing body load quickly yielding a sense of body-orgasm. No CEV's, slight fractal visual distortions with open eyes. Peak effects 5-25 mins. Total time to full baseline 1.5-2.0 hours
Medium doses (7-15 mgs smoked) - Crushing body load yielding gasping and gagging nausea with any resistance and a sort of release into the 5-meo state with surrender - beyond body-orgasm into gasping soul orgasm. CEV's show a sort of yellow or white field. Eyes want to open and close. Open eye visuals are best with living, loved ones. Fractalized in space and time. Peak effects 20-40 mins. Total time to full baseline 1.5-2.0 hours.
Higher doses (16-22 mgs smoked) - Body load that hits like a wall of drowning whiteness. Overwhelming. No time to resist or submit or feel nauseaus or groan or . . . Blackout for peak memories. Weird verbal outbursts or babbling. Extended peak effects, 20 mins to over an hour. Total time to baseline 2 hours to . . . . extended feeling of not being fully back or integrated that can last days, weeks or months.
Pharmacology, toxicity and general safety
When ingested together with a MAOI, 5-MeO-DMT is O-demethylated by CYP2D6 and becomes Bufotenine in the liver, but without a MAOI, it is mostly N-Demethylated by MAOI into 5-MeO-Indole-acetic-acid (Shen et al 2010; Ai-Ming 2008).
There is some concern regarding taking 5-MeO-DMT orally, specially with a MAOI. There has been at least one reported death (Sklerov, 2005; Callaway 2006), and some hospitalizations. This might be due to individual metabolic differences. Check this thread for more info. If you are consuming 5-MeO-DMT orally for the first time, specially with MAOIs, start VERY low and raise your dosage gradually.
General Health and Safety tips regarding set and setting, how to integrate and so on, as written for DMT, are also relevant to 5-MeO-DMT
Plants containing 5-MeO-DMT
The alkaloid information about Acacia coming from TLC analysis is not to be considered definite. They are tentative identifications that need to be confirmed with GC-MS or other appropriate analytical methods.
- Traces 5-MeO-DMT tentatively observed in twigs. 5 Oct. 1995. TLC by J. Appleseed 1995 (Xanthydrol), co-occuring with suspected DMT. (Trout's Notes)
Acacia angustissima Trace amounts tentatively observed in roots (unconfirmed) in March 1995. TLC by J. Appleseed. Not ob- served in second assay. Trace amounts in seeds. tic by Appleseed 1995 (all with Xanthydrol)
Acacia auriculiformis Trace amounts tentative ly ()bserved in stem-bark (25 April 1995). tic by J. Appleseed 1995 (A band at this Rfwas a lso seen in roots [mislabeled as Guaiacuml in 2 Sept. 1994 assay but used Ehrlichs reagent which does not differentiate between DMT and 5-McO- DMT.)
Acacia cultriformis Blue band co-chromatographing with 5-MeO-DMT (Xanthydrol) Seen in branch stems, and in ph yllodes, also in flowering spikes. Commercial florist's material. Sept 1996.
Blue band co-chromatograpbing with 5-MeO-DMT (Xanthydrol) Roots of two year old seedlings. lie by J. Appleseed 1996. A Iso in stems Sept 1996. Not observed in roots Sept. 1996.
Acaciafarnesiana Traces tentatively observed in green fruit. Not present in ripe fruit. (Xanthydrol) Also contained a suspected f3-carboline. (blue under UV) 24 July 1995. tic by J. Appleseed 1995.
Acacia maidenii Traces observed in wood (October 1995); Observed in twigs (26 July in phyllode and in mixed phyllode and twigs 27 Oct 1995) tic by J. Applcseed 1995 (Xanthydrol) Co-occurring with suspected DMT in all samples of phyllodesltwig but sole alkaloid seen when just using twigs. Not observed in bark or root bark. [Note: Acacias such as this one do not produce leaves except when young or stressed. What are thought of as being leaves are actually phyllodes; a specially modified petiole (the short stalk at the leaf base.)]
Acacia nilorica
Faint traces observed in stem, roots and leaves. As-
sayed separately {Sept 1996; Xanthydrol)
Acacia obtusifolia
In some samp les as a minor component. Apparently
unpttblished work. Pers. comm. with Snu Voogelbre-
inder. Needs coufrrmation.
Appeared present in one sample ofl~avesltwigs looked
at by Mulga: Not observed in bark or root bark.
Acacia victoriae Strong blue band eo-chromatographing with 5-McO- DMT (Xanthydrol) Roots of two year old seedlings. tic by J. Appleseed 1996.
Extraction teks
Any typical extraction teks for DMT should potentially extract 5-MeO-DMT (but it will also extract DMT together).
Dosages and consumption methods
5-MeO-DMT is around 5 times more potent than DMT by weight.
Smoked, dosage is around 5-15mg.
Oral, according to Jonathan Ott, 30mg are active without the need for MAOIs. Mixing with MAOIs, it will be 3 times stronger, so a dosage will be around 10mg. There is some concern about 5-MeO-DMT's oral ingestion safety, and it might be related with individual metabolism differences. Check this thread for more info. If you are consuming 5-MeO-DMT orally for the first time, specially with MAOIs, start VERY low and raise your dosage gradually.
Insufflated, 5-20mg (Erowid)
History of usage
Analysis of 5-MeO-DMT
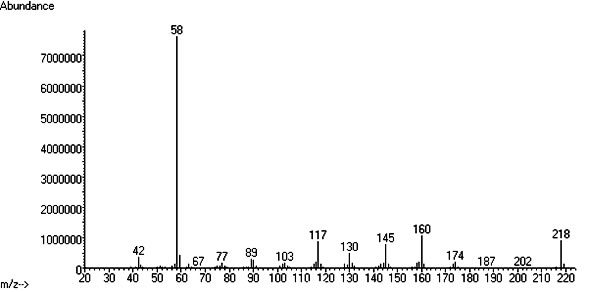
GC-MS
Retention time: 12.946 (System used)
Other info: 5-MeO-DMT: EI/MS (m/z, %): 218 (M+ , 10), 160 (6.3), 145 (2.5), 117 (5.0), 58 (100), 42 (4). (Takahashi 2008)
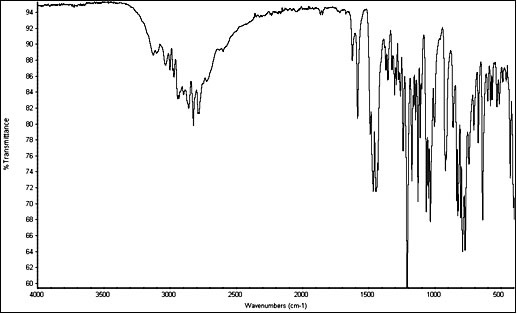
InfraRed
5-MeO-DMT freebase
NMR
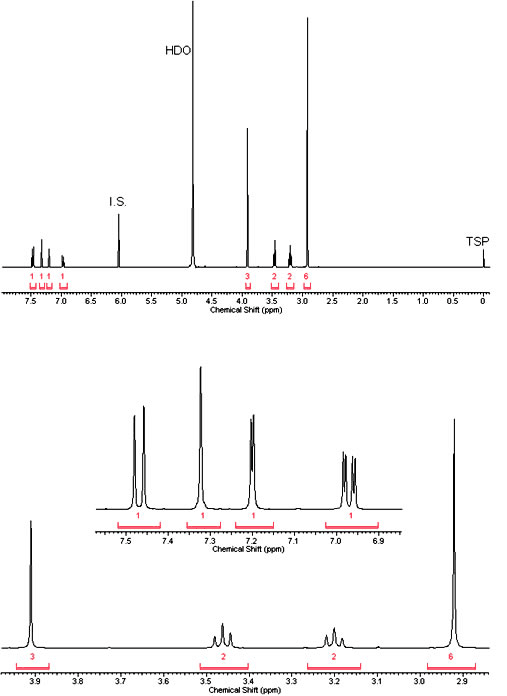
1H NMR (400 MHz, D2O) δ ppm 7.47 (d, J=8.90 Hz, 1 H) 7.32 (s, 1 H) 7.20 (d, J=2.45 Hz, 1 H) 6.97 (dd, J=8.90, 2.45 Hz, 1 H) 3.91 (s, 3 H) 3.46 (t, J=7.43 Hz, 2 H) 3.20 (t, J=7.43 Hz, 2 H) 2.92 (s, 6 H).
(Source: Microgram Bulletin Vol. 3, Pg 8)
Other info: 1 H-NMR (CDCl , δ): 2.34 (6 H, s), 2.63 (2 H, m), 2.91 (2 H, m), 3.86 (3 H, s), 6.85 (1 H, dd, J = 2.3, 8.6 Hz), 6.98 (1 H, br d, J = 2.3 Hz), 7.05 (1 H, d, J = 2.3 Hz), 7.22 (1 H, d, J = 8.6 Hz). 13 C-NMR (CDCl3 , δ): 23.7, 45.4, 56.0, 60.1, 100.8, 111.8, 112.1, 113.9, 122.3, 127.8, 131.5, 153.9. (Takahashi 2008)