Mescaline
Contents
- 1 Brief overview - What is Mescaline?
- 2 Chemical and physical properties
- 3 Effects
- 4 Pharmacology, toxicity and general safety
- 5 Plants containing mescaline
- 6 Extraction teks
- 7 Dosages and consumption methods
- 8 History of usage
- 9 Analysis of Mescaline
- 10 Scientific publications
- 11 Other links of interest
Brief overview - What is Mescaline?
Chemical and physical properties
Effects
Pharmacology, toxicity and general safety
Mescaline
3,4,5-trimethoxyphenethylamine
Freebase Mescaline
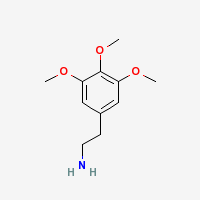 |
- CAS Registry Number: 54-04-6
- Composition: C11H17NO3
- Melting point: 35-36°C (Kindler and Peschke, 103. Merck Index)
- Boiling point: 180°C (12 mmHg)
- XLogP: 0.6 (PubChem)
- XLogP3: 0.7 (PubChem)
- pKa: 9.56
- Appearance: long needle shaped white crystals
- Molecular weight: 211.26
Notes: forms mescaline carbonate on prolonged exposure to air
- Average dose: 300 to 600 milligrams with a duration of 5 to 12 hours.
- Colorimetric reagent results: Here
- Solubility
Soluble in: alcohol, chloroform, benzene, xylene, toluene, acetone, dichloromethane, highly soluble in isopropyl alcohol, soluble in d-limonene
Moderately soluble in: water
Insoluble in: practically insoluble in ether or petroleum ether
- LD50: i.p. rats 370 mg/kg
Mescaline Citrate
- Solubility
Soluble in water
Insoluble in: xylene, acetone *
* Possible unreliable web source.
Mescaline Hydrochloride
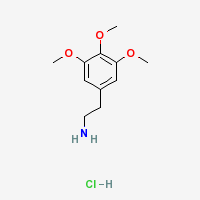 |
- Molecular weight: 247.72 (Sigma Aldrich)
- Empirical Formula (Hill Notation): C11H17NO3 • HCl (Sigma Aldrich)
- CAS Number: 832-92-8
- Appearance: colorless crystals, needles
- Melting point: 184°C (Merck Index)
- Solubility:
Moderately soluble in: water, alcohol (Merck Index), methanol (at least 1.0 mg/ml, source Sigma Aldrich solution) (Merck Index) Insoluble in: practically insoluble in toluene and acetone, insoluble in isopropyl alcohol, diethyl ether, and d-limonene
- LD50: i.p. rats 132 mg/kg
- Storage temperature: 2-8°C (Sigma Aldrich)
- Isolation: when mescaline hydrochloride is extracted from San Pedro, Achuma, or Peruvian torch, it can be isolated from the other alkaloids by washing it in IPA or acetone (use 10 ml per gram of alkaloids, and wash 2-3 times). The non-mescaline alkaloids dissolve in the IPA or acetate, while the mescaline hydrochloride does not. Note that for the cleanest results use about 2 washes of acetone, and then 2 washes with IPA.
Mescaline Picrate
- Melting point: mp 222°C.
Mescaline Sulfate Dihydrate
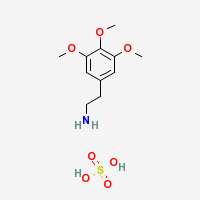 |
- Composition: (C11H17NO3)2 • H2SO4 • 2H2O
- Appearance: prisms
- Melting point: 183–186 °C (361–367 °F)
- Molecular Weight: 309.33606
- Soluble in: hot water, methanol
- Almost insoluble in: near freezing water, alcohol, acetone *
* Possible unreliable web source.
Mescaline Fumarate
- Composition: unknown (it may be a one to one salt or may not be)
- Appearance: White powder
- Melting point: unknown
- Solubility
Soluble in: water
Insoluble in: Limonene, Anydrous IPA, Acetone, MEK (most likely insoluble in all non-polars like xylene and toluene) Source
Mescaline Acetate
- Composition: unknown
- Appearance: white free flowing powder with a slight waxy texture ***
- Melting point: unknown
- Solubility:
Soluble in: water***, isopropyl alcohol***, acetone***, DMSO*** (more than 5 grams/100 ml), boiling MEK***
Insoluble in: xylene, d-limonene, cold MEK (Methyl Ethyl Ketone) ***
*** This information was validated by SWIM and is reliable.
- Isolation: when mescaline acetate is extracted from San Pedro, Achuma, or Peruvian torch, it can be isolated from the other alkaloids by washing it in cold MEK (use 10 ml per gram of alkaloids, and wash 2-3 times). The non-mescaline alkaloids dissolve in the MEK, while the mescaline acetate does not. Mescaline acetate can be recrystallized in MEK by boiling the MEK and then freezing it overnight.