CIELO
Contents
Introduction
CIELO stands for Crystals In Ethyl-acetate Leisurely OTC (Over The Counter).
In this TEK, aqueous cactus paste is broken down with microwave radiation, basified with lime, made clumpy with CaCl2, and extracted with ethyl acetate. The extract is salted with citric acid to precipitate mescaline citrate crystals directly in the solvent.
Materials
- Quart jars with LDPE or PP plastic lids (e.g. below)
- Food scale
- 300g water
- 100g powdered dry cacti
- Microwave
- 25g Ca(OH)2 (lime)
- 25g anhydrous CaCl2
- ~ 1000g ethyl acetate ("MEK substitute")
- pH paper
- Citric acid
- Filter (optional)
- Shallow baking dish
- Scraping tool (e.g. razor blade)
Safety
Review ethyl acetate's safety information[1] and check the manufacture's MSDS to verify you have pure ethyl acetate.
Each adult individual needs to find and review any other relevant safety information throughly and make their own personal decision on proceeding.
Process
Paste
Mix water and cactus powder in a a quart jar. Microwave in short bursts, interrupting the irradiation so the paste does not swell over the rim. This can get to be only a few seconds, so patience is required. Stir frequently and keep track of evaporation with a food scale. Paste color will change from green to tan. Once ~55g of water evaporate microwaving is complete.
Mix in lime until homogeneous and then mix in CaCl2 until paste breaks up into spongy clumps.
With a little practice this step can be done in under an hour, but it does require some elbow grease. See image below from initial green water paste and final clumpy tan paste.
Pull
Add ~ 200g of ethyl acetate to the paste making the jar ~3/4 full and seal with proper lid. Extract for at least 20 minutes regularly shaking every once in a while. Allow to rest and settle for at least 15 minutes and decant to a second jar.
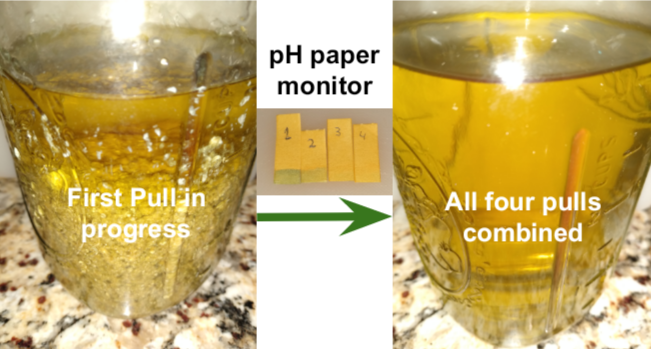
Pull three more times. Progress can be monitored with pH paper (green color indicates free base presence - see middle of image bellow). Paste will change during the pulls and become gunky. When working with a gunky paste, stir, don't shake.
Combined pulls will give ~a quart of clear yellow extract (see image below). Optionally, more pulls can be done into a different jar for a modest yield improvement.
Salt
Drop ~250mg (~1/16 tsp) of citric acid into the extract making it cloudy and let it passively dissolve and react (12 to 48h) in the fridge until clear with crystals on the jar surfaces. Test with pH paper, salting is complete when pH paper is acidic. If needed, add more citric acid in small increments of ~100mg.
Finish crystalization in the freezer to maximize yield.
If there are issues with xtalization, it should still be possible to recover any product present with water pulls followed by evaporation and ethyl acetate washes.
Finish
Decant ethyl acetate into a storage jar for reuse, using a filter to catch any loose crystals. Rinse crysals with fresh ethyl acetate at least once or until yellow color is removed to personal cosmetic satisfaction. Dissolve crystals in minimal warm water and passively evaporate undisturbed in a shallow baking dish. Finally, scrape up the resulting long crystal needles.
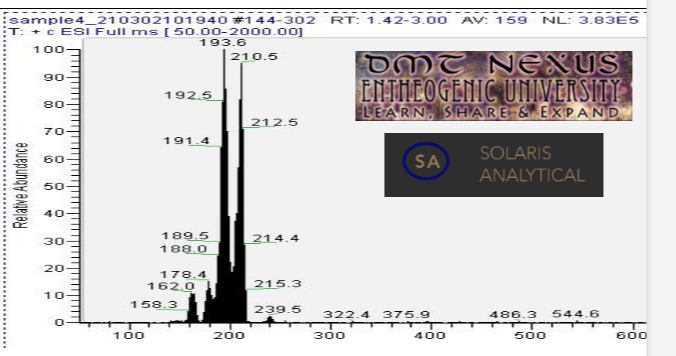
Mass spectrometry (MS) results from solaris analytical[2] indicate the product is very clean mescaline after only two ethyl acetate rinses with some minor discoloration remaining. See MS spectrum below, peak near 210.5 is mescaline. Peaks at and 193.6, 178.4, and 162.0 are believed to be mescaline with amine/methyl/methoxy groups cleaved to generate the lower mass mescaline spectrum in multiples of ~16 au (16.9, 32.1, and 48.5 respectively). The small peak at 239.5 is not attributed to mescaline.
Lab Notes
The microwave radiation breaks down the plant matter potentially helping free mescaline from the biological matrix. CaCl2 improves the paste texture and helps keep water out of the solvent. CaCl2 can lower the pH by making Ca(OH)2 less soluble, however pH paper indicates paste is still alkaline. Overall, the resulting plant paste seems to give give efficient and clean pulls that are ready to to crash the mescaline salt. Different paste process order, skipping the microwave, using NaCl instead of CaCl2, or using acetone instead of ethyl acetate, could work too, but may have lower yields, produce a less workable paste, or carry unwanted plant matter that interferes with crystalization or ends up in the product.
Allowing 8 hours rest before decanting gives the pull enough time for ethyl acetate, water, and cactus/Ca(OH)2/CaCl2 time to settle and reach liquid-liquid equilibrium.
It is possible to chemically dry the extract with a drying agent such as anhydrous MgSO4. However, no clear yield benefit was observed by performing this step. Surprisingly, solutions carefully dried with anhydrous CaCl2 followed by K2CO3 had difficulty crystalizing after salting, indicating that a some water is desirable for crystalization. This points to the precipitate from this TEK maybe being hydrate salt. Regardless of the reason, the small water content in ethyl acetate directly from the pulls is in a good range experimentally.
During salting, every 10mg of citric acid (CitH3) reacts with enough free base mescaline (Mes) to precipitate up to 43mg of mescaline citrate (or slightly more if a hydrate form is precipitating):
250mg of citric acid are enough for the typical cactus (up to 1% yield). However, and outlier like the legendary Ogun would need ~1100mg of citric acid for a 4.7% yield.
Over acidifying is not a big concern. There is room for excess citric acid in solution since several grams can dissolve in a quart of ethyl acetate even when chilled. The vast majority of excess acid would be poured off after salting, and any traces removed when washing the crystals.
Other solid organic acids could work. Fumaric acid was tested and gave wispy tiny crystals (not ideal). Malic was tested and slowly crystalized from a dry solution. Tartaric could not crystalize well from dry a dried extract, but began to crystalize with some water added (similar to citric acid). There are many other solid organic acids soluble in ethyl acetate that can be tested (ascorbic, succinic, etc).
It is possible that some of the assumptions and conclusions in these lab notes are incorrect or incomplete. The process was simply tuned for good yields, but the search was not exhaustive[3]. There could be ways to improve this process.
References
Shroombee's Notes
Experiment #1
March 9, 2021
6:42 pm
100 grams Peruvian Torch chips
298 grams purified water
Blended cactus chips in the Vitamix dry container so it becomes a fine powder.
6:58 pm Mixed cactus powder and water in a plastic mixing bowl rather than a mason jar because it seems easier. Product is a fluffy texture, olive green color. There is no excess water.
The bowl plus cactus plus silicone mixing spoon weighs 826.5 grams. Microwave on high for 30 seconds, mix, then weigh. This is pretty easy. I wouldn't want to do this in a mason jar. The cactus does not bubble and there is no issue at 30 seconds with anything bubbling over. Weight after each 30 second cycle:
825.6 grams
823.9 grams
820.2 grams
816.0 grams
808.7 grams
800.7 grams
791.3 grams
783.2 grams
775.8 grams
765.8 grams
DONE
After a couple of the microwaving cycles, the cactus lost its fluffiness and became like light bread dough, not sticking much to the bowl.
7:25 pm Begin slowly mixing in 25 grams pickling lime. Started with the plastic bowl but switched to a ceramic bowl after a minute, not knowing how the plastic would react to the base. Bowl appears fine after washing it out.
7:39 pm I'm using a fork to try to get the lime mixed evenly. I figure my Kitchenaid stand mixer will be easier so I break that out.
7:45 pm Letting the Kitchenaid stir the cactus for me. Much easier!
7:53 pm Added 25 grams calcium chloride. Before adding, the cactus paste was fluffy and stuck to the sides of the stainless steel bowl. After adding the calcium chloride, the cactus became like clumpy sand and hardly stuck to the sides of the bowl.
7:58 pm Stopped mixing.
8:05 pm Transferred cactus to a wide mouth quart mason jar and added 220 ml of ethyl acetate (weighing 199.9 grams). The ethyl acetate came up to approximately 540 ml on the side of the mason jar. After shaking, the cactus looked like fluffy beach sand.
8:28 pm Shake for a minute.
9:00 pm Shake for a minute.
9:22 pm No shaking, I notice the ethyl acetate is light green.
10:30 pm Shake for a minute. The cactus is starting to get a little stickier, leaving streaks on the glass.
11:00 pm Ethyl acetate comes up to exactly 500 ml on the side of the mason jar. Versus ~540 ml at 8:05 pm when the ethyl acetate was first added. What accounts for this 40 ml difference?
12:00 am Shake for a minute.
1:06 am Weighed mason jar with cactus: 1001.4 grams.
March 10, 2021
7:57 am Weighed mason jar with cactus: 1001.1 grams.
Ready to decant ethyl acetate. Large pyrex beaker with metal coffee filter weighs 342.3 grams. Ethyl acetate is a medium, emerald green. Definitely not light green and not yellow.
Using a small ladle to decant, which gets most of the ethyl acetate with no plant matter. Beaker weighs 427.2 grams meaning we recovered 84.9 grams of ethyl acetate. Not too good.
I transferred the cactus to a french press to see if I could recover more ethyl acetate. The sticky paste does not compress and I recovered an additional ~6 grams ethyl acetate. French press is obviously not worth the effort!
8:21 am Done with decanting, transferring, french pressing, weighing, et cetera. Total recovery for this pull is 90.6 grams ethyl acetate.
8:23 am Added 90.7 grams of fresh ethyl acetate to the mason jar. Stirred ethyl acetate into the sticky cactus, did not shake. Ethyl acetate is already changing to green color.
8:33 am Photo of pH paper. Paper is dark green. pH 10 or 11? Also tested using the 4 color pH strips. More difficult to judge the pH with these.
8:34 am Photo of recovered ethyl acetate showing it is an emerald green color.
8:45 am Poured 10 ml of ethyl acetate into a small beaker. Added 13 mg of citric acid. Lower half of the beaker turned milky, cloudy. Checked pH and it's basically the same as the rest of the solvent. So the small amount of citric acid didn't change the pH much. I used a disposable pipette and pulled from the top of the solvent, so perhaps the top layer didn't have a chance to react yet.
8:49 am Photo showing cloudy 10 ml in small beaker.
8:50 am Poured the 10 ml and the rest of the solvent into a mini Pyrex baking dish, using the larger volume of solvent to rinse out of the small beaker. I probably should have left the solvent in a mason jar or beaker rather than adding to the baking dish. Added 95 mg of citric acid. Stirred a little because I wanted to get a more accurate pH. pH was about 7. Added an additional 61 mg citric acid. pH went to about 6. Total citric acid added to the 90.6 grams ethyl acetate is 169 mg. Even after rinsing with the main solvent, the bottom of the small beaker has some spots that look like something crystalizing. I don't know if it's just sticky citric acid or ???
9:07 am Photo of 3 pH test strips.