Difference between revisions of "CIELO"
(→Lab Notes) |
(→Pull) |
||
| Line 48: | Line 48: | ||
| − | Pull three more times. Progress can be monitored with pH paper (green color indicates free base presence). Paste will change during the pulls. If solvent becomes trapped in a gunky paste, adding CaCl2 should free it up. | + | Pull three more times. Progress can be monitored with pH paper (green color indicates free base presence). Paste will change during the pulls. If solvent becomes trapped in a gunky paste, adding CaCl2 should free it up and stir, don't shake anymore. |
Revision as of 16:48, 8 March 2021
Contents
Introduction
CIELO stands for Crystals In Ethyl-acetate Leisurely OTC (Over The Counter).
In this TEK, aqueous cactus paste is broken down with microwave radiation, basified with lime, made clumpy with CaCl2, and extracted with ethyl acetate. The extract is salted with citric acid to precipitate mescaline citrate crystals directly in the solvent.
Materials
- Quart jars with LDPE or PP plastic lids (e.g. below)
- Food scale
- 300g water
- 100g powdered dry cacti
- Microwave
- 25g Ca(OH)2 (lime)
- 25g anhydrous CaCl2
- ~ 1000g ethyl acetate ("MEK substitute")
- pH paper
- Citric acid
- Filter (optional)
- Shallow baking dish
- Scraping tool (e.g. razor blade)
Safety
Review ethyl acetate's safety information[1] and check the manufacture's MSDS to verify you have pure ethyl acetate.
Each adult individual needs to find and review any other relevant safety information throughly and make their own personal decision on proceeding.
Process
Paste
Mix water and cactus powder in a a quart jar. Microwave in short bursts, monitoring closely to interrup the irradiation to stop the paste from swelling over the jar. Stir frequently and keep track of evaporation with a food scale. Paste color will change from green to tan. Once ~50g of water evaporate microwaving is complete.
Mix in lime until homogeneous and then mix in CaCl2 until paste breaks evenly up into small spongy clumps.
Pull
Add ~ 200g of ethyl acetate to the paste making the jar ~3/4 full and seal with proper lid. Extract for a few hours regularly shaking for several minutes. Allow to rest overnight and decant extract to a second jar.
Pull three more times. Progress can be monitored with pH paper (green color indicates free base presence). Paste will change during the pulls. If solvent becomes trapped in a gunky paste, adding CaCl2 should free it up and stir, don't shake anymore.
Combined pulls will give ~a quart of clear yellow extract. Optionally, more pulls can be done into a different jar for a modest yield improvement.
Salt
Dissolve ~250mg (~1/16 tsp) of citric acid into the extract making it cloudy. Test with pH paper. Salting is complete when pH paper is acidic. If needed, add more citric acid in small increments of ~100mg.
Place extract in fridge to fully crash mescaline citrate crystals over 12-72 hours. Precipitation is complete when salted extract goes from cloudy to clear.
If crystals do not form after a few days there was an issue with the TEK. A freezer precipitation can be tried. If that doesn't work either, it should still be possible to recover any product in the solvent with water pulls followed by evaporation and ethyl acetate washes.
Finish
Decant ethyl acetate into a storage jar for reuse, optionally using a filter if any loose crystals pour out. Rinse crysals with fresh ethyl acetate at least once. Optionally, continue rinsing until yellow color is removed to personal cosmetic satisfaction. Leave xtals uncovered to evaporate all residual solvent. Dissolve crystals in minimal warm water and passively evaporate undisturbed in a shallow baking dish to obtain long crystal needles and scrape them up.
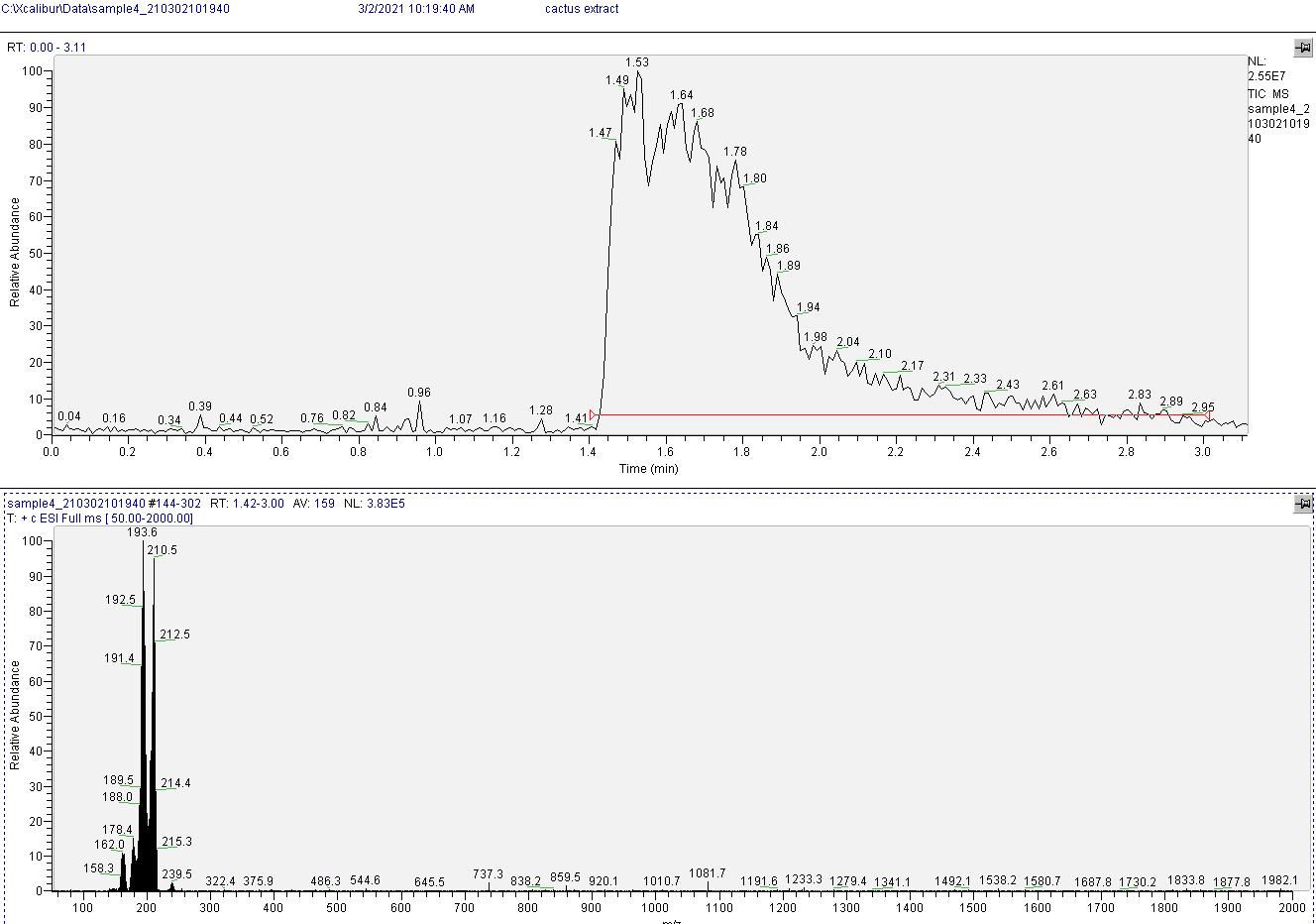
Mass spectrometry results indicate the product is very clean mescaline. See image below, peaks near 210 and 194 are from mescaline (second peak is from mescaline's amine cleavage during measurement).
Lab Notes
The microwave radiation breaks down the plant matter. CaCl2 improves the paste texture and helps keep some water out of the extract. CaCl2 can lower the pH by making Ca(OH)2 less soluble, however a test 250g water solution with 25 of Ca(OH)2 and 25g of CaCl2 had a pH of 11.1, above mescaline's pKa of 9.6. Overall, the resulting plant paste seems to give give efficient and clean pulls ready to to crash the mescaline salt. Different paste process order, skipping the microwave, using NaCl instead of CaCl2, or using acetone instead of ethyl acetate, could work too, but may have lower yields, produce a less workable paste, or carry unwanted plant matter that interferes with crystalization or ends up in the product.
Allowing 8 hours rest before decanting gives the pull enough time for ethyl acetate, water, and cactus/Ca(OH)2/CaCl2 time to settle and reach liquid-liquid equilibrium.
It is possible to chemically dry the extract with a drying agent such as anhydrous MgSO4. However, no clear yield benefit was observed by performing this step. Surprisingly, solutions carefully dried with anhydrous CaCl2 followed by K2CO3 had difficulty crystalizing after salting, indicating that a some water is desirable for crystalization. Experimentally, the water content in ethyl acetate from this TEK is in a good range.
During salting, every 10mg of citric acid (CitH3) reacts with enough free base mescaline (Mes) to precipitate up to 43mg of mescaline citrate:
250mg of citric acid are enough for the typical cactus (up to 1% yield). However, and outlier like the legendary Ogun would need ~1100mg of citric acid for a 4.7% yield.
Over acidifying is not a big concern. There is room for a lot of excess citric acid in solution since several grams can dissolve in a quart of ethyl acetate even when chilled.
Other solid organic acids could work. Fumaric acid was tested and gave wispy tiny crystals (not ideal). Malic would be interesting since that could yield the (reportedly?) natural mescaline salt form. There are many other solid organic acids soluble in ethyl acetate that can be tested (tartaric, ascorbic, succinic, etc).
It is possible that some of the assumptions and conclusions in these lab notes are incorrect or incomplete. The process was simply tuned for good yields, but the search was not exhaustive. There could be ways to improve this process.