Difference between revisions of "Chilled Acetone with IPA and Naphtha"
(→Salt) |
(→Finish) |
||
| Line 65: | Line 65: | ||
=== Finish === | === Finish === | ||
| − | Pour off ethyl acetate into a storage container for reuse. A filter can help catch any crystals that accidentally pour out if needed. Rinse xtals with | + | Pour off ethyl acetate into a storage container for reuse. A filter can help catch any crystals that accidentally pour out if needed. Rinse xtals with ethyl acetate at least once. Optionally, continue rinsing until yellow color is removed to personal cosmetic satisfaction. Leave xtals uncovered to evaporate all residual solvent, this is the final product. If desired, the product can be dissolved in minimal warm water and passively evaporated undisturbed in a shallow baking dish to obtain long crystal needles that are easy to scrape. |
| Line 71: | Line 71: | ||
[[File: Cactus-extract.jpg]] | [[File: Cactus-extract.jpg]] | ||
| − | |||
== Lab Notes == | == Lab Notes == | ||
Revision as of 01:41, 8 March 2021
Contents
Introduction
CIELO stands for Crystals In Ethyl-acetate Leisurely OTC (Over The Counter).
In this TEK, aqueous cactus paste is broken down with microwave radiation, basified with lime, made clumpy with CaCl2, and extracted with ethyl acetate. The extract is salted precipitating mescaline citrate crystals in the freezer.
Materials
- Quart jars with LDPE or PP plastic lids (e.g. below)
- Food scale
- 300g water
- 100g powdered dry cacti
- Microwave
- 25g Ca(OH)2 (lime)
- 25g CaCl2
- ~ 1000g ethyl acetate ("MEK substitute")
- pH paper
- Citric acid
Safety
Review ethyl acetate's safety information[1] and check the manufacture's MSDS to verify you have pure ethyl acetate.
Each adult individual needs to find and review any other relevant safety information throughly and make their own personal decision on proceeding.
Process
Paste
Mix water and cactus powder in a a quart jar. Microwave in short bursts (avoid swelling over). Stir frequently and keep track of evaporation with a food scale. Paste color will change from green to tan. Once ~50g of water evaporate microwaving is complete.
Mix in lime until homogeneous and then mix in CaCl2 until paste breaks up into small spongy chunks.
Pull
Add ~ 200g of ethyl acetate to the paste making the jar ~3/4 full and seal with proper lid. Extract for a few hours regularly shaking for several minutes. Decant extract to a second jar.
Pull three more times. Progress can be monitored with pH paper (green color indicates free base presence). Paste will change during the pulls. If solvent becomes trapped in a gunky paste, adding CaCl2 should free it up.
Combined pulls will give ~a quart of clear yellow extract. Optionally, more pulls can be done into a different jar for a modest yield improvement.
Salt
Add ~250mg (~1/16 tsp) of citric acid into the extract. After it dissolves, test with pH paper. Salting is complete if pH paper is acidic. If needed, add citric acid until extract becomes acidic.
Place extract in freezer to fully crash mescaline citrate crystals over 24-72 hours. Precipitation nos complete when salted extract goes from cloudy to clear.
If crystals do not form after a few days there was an issue with the TEK. It should still be possible to recover any product in the solvent with water pulls followed by evaporation and ethyl acetate washes.
Finish
Pour off ethyl acetate into a storage container for reuse. A filter can help catch any crystals that accidentally pour out if needed. Rinse xtals with ethyl acetate at least once. Optionally, continue rinsing until yellow color is removed to personal cosmetic satisfaction. Leave xtals uncovered to evaporate all residual solvent, this is the final product. If desired, the product can be dissolved in minimal warm water and passively evaporated undisturbed in a shallow baking dish to obtain long crystal needles that are easy to scrape.
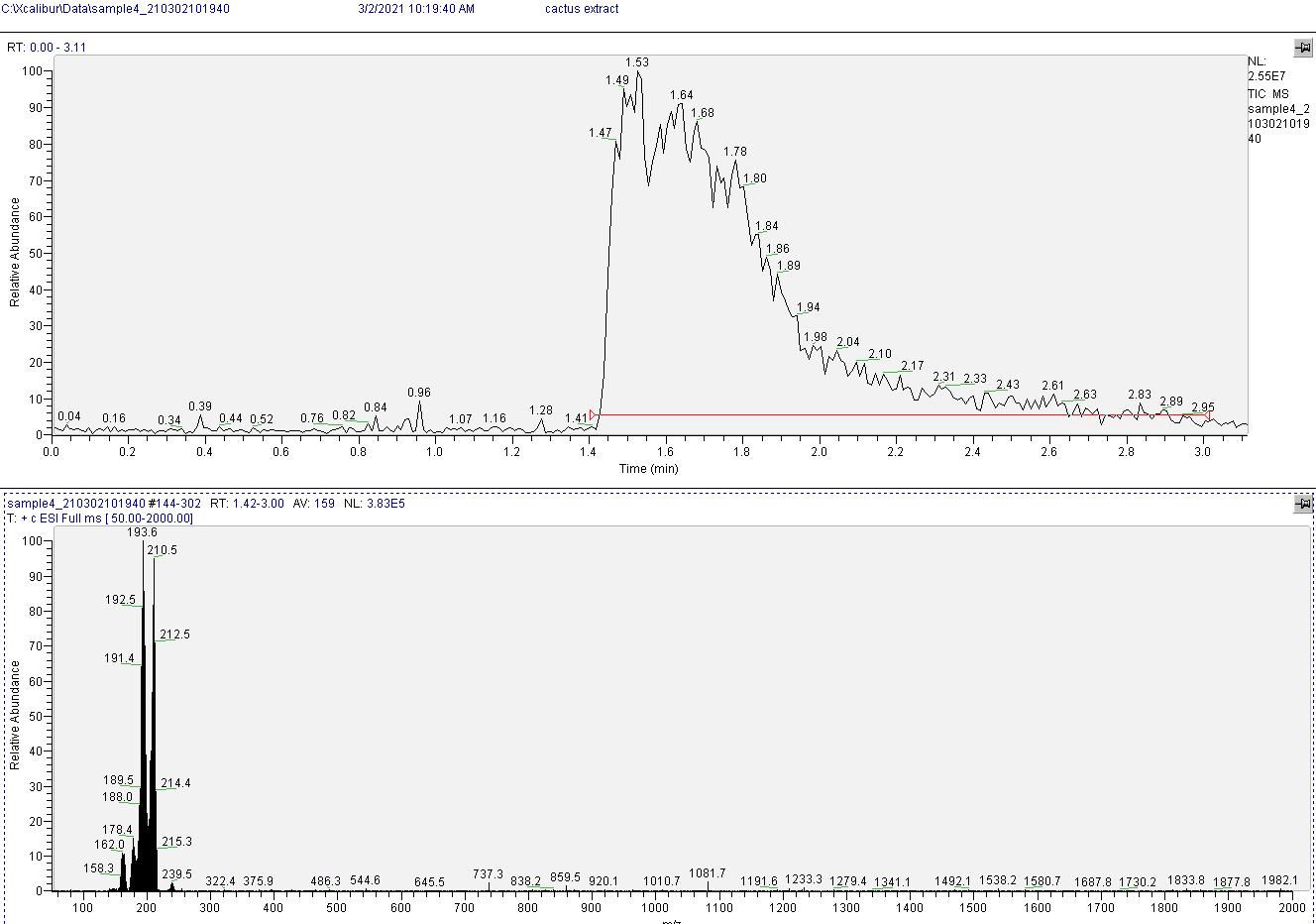
Mass spectrometry results indicate the product is very clean mescaline. See image below, peaks near 210 and 194 are from mescaline (second peak is from amine cleavage during measurement).
Lab Notes
The microwave radiation breaks down the plant matter which gives both efficient and clean pulls that are a good environment to crash the mescaline salt. Different paste process order, skipping the microwave, using NaCl instead of CaCl2, or using acetone instead of ethyl acetate, can work too, but may have lower yields and/or produce a less workable paste.
After the pulls, it is possible to dry the extract over anhydrous MgSO4. However, no clear yield benefit was observed from this step. It appears the water content in ethyl acetate from the alakaline CaCl2 paste is low enough to fully precipitate mescaline citrate when cold.
During salting, every 10mg of citric acid(CitH3) reacts with enough free base mescaline (Mes) to precipitate up to 43mg of mescaline citrate:
250mg of citric acid are enough for the typical cactus (up to 1% yield). However, and outlier like the legendary Ogun would need ~1100mg of citric acid for a 4.7% yield.
Over acidifying is not a big concern. There is room for a lot of excess citric acid in solution since several grams can dissolve in a quart of ethyl acetate.
Other solid organic acids could work. Fumaric acid was tested and gave wispy tiny xtals (not ideal). Malic would be interesting since that could yield the (reportedly?) natural mescaline salt form. There are many other solid organic acids soluble in ethyl acetate that can be tested (tartaric, ascorbic, succinic, etc).
It is possible that some of the assumptions and conclusions in these lab notes are incorrect or incomplete. The process was simply tuned for good yields, but the search was not exhausted. There could be ways to improve this process.