Difference between revisions of "Solvent physical properties"
From DMT-Nexus Wiki
Mindlusion (Talk | contribs) (→Acetic acid) |
Mindlusion (Talk | contribs) (→Formic acid) |
||
| Line 40: | Line 40: | ||
====Formic acid==== | ====Formic acid==== | ||
| + | [[File:Formicacid.png]] | ||
{| class="wikitable" style="text-align: center; color: green;" | {| class="wikitable" style="text-align: center; color: green;" | ||
!Solvent | !Solvent | ||
Revision as of 06:01, 14 December 2012
page in progress....
Contents
Solvents
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| Acetone | 56 °C | Miscible | 0.786 g/ml | 58.08 g/mol |
Note about Density: If the density of the solvent is greater then one (mass > 1.00) the solvent, if non-polar will sink below water.
likewise if the density is less then one (mass < 1.00) the solvent, if non-polar, will float on the surface of the water
Polar protic
Water
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| Water | 100 °C | Miscible | 1.000 g/ml | 18.02 g/mol |
Carboxylic acids
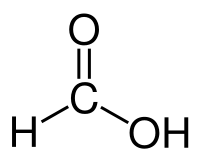
Formic acid
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| Formic acid | 100.8 °C | Miscible | 1.22 g/ml | 46.03 g/mol |
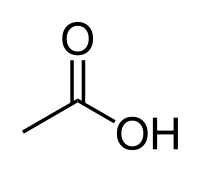
Acetic acid
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| Acetic acid | 118 °C | Miscible | 1.049 g/ml | 60.05 g/mol |
Alcohols
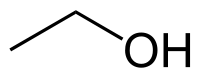
Ethanol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C2H5OH | 79 °C | Miscible | 0.789 g/ml | 46.07 g/mol |
Azeotrope % weight /w water = 95.5%
Miscible in:
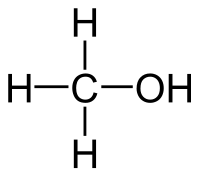
Methanol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C2H5OH | 65 °C | Miscible | 0.791 g/ml | 32.04 g/mol |
No azeotrope w/ water
Miscible in:
Propanol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C2H5OH | 97 °C | Miscible | 0.803 g/ml | 60.1 g/mol |
Azeotrope % weight /w water = 71.7%
Miscible in:
Isopropanol
Glycerol
Propylene Glycol
Polar aprotic
Ketones
Short chain ketones are miscible in ALL organic solvents
Acetone
| Formula | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C3H6O | 56 °C | Miscible | 0.786 g/ml | 58.08 g/mol |
Miscible in:
All organic solvents
- Does not from an Azeotrope with water
Methyl ethyl ketone (MEK)
| Formula | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C4H8O | 80 °C | Miscible 275. g/L | 0.805 g/ml | 72.11 g/mol |
Miscible in:
All organic solvents
Cyclohexanone
| Formula | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C6H10O | 155.65 °C | Miscible 90 g/L | 0.9478 g/ml | 98.15 g/mol |
Miscible in:
All organic solvents
Esters
Ethyl acetate
Ethers
Tetrahydrofuran
Others
Dimethyl sulfoxide (DMSO)
Acetonitrile
Dimethylformamide
Non-polar
Aliphatic
Butane
Pentane
Hexane
Heptane
Octane
Cyclic
Cyclohexane
Cyclopentane
Aromatic
Benzene
Toluene
Xylene
Ethers
Diethyl ether
Chlorinated hydrocarbons (only slighlty polar)
Chloroform
Carbon Tetrachloride
Dichloromethane (DCM)
Other
d-limonene
Common Azeotropes
(taken from wikipedia)
Azeotropes of water, b.p.=100 °C
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cite error: <ref> tags exist, but no <references/> tag was found