Difference between revisions of "5-MeO-DMT"
Endlessness (Talk | contribs) (→Pharmacology, toxicity and general safety) |
Endlessness (Talk | contribs) (→Pharmacology, toxicity and general safety) |
||
| Line 16: | Line 16: | ||
== Pharmacology, toxicity and general safety == | == Pharmacology, toxicity and general safety == | ||
| − | When ingested together with a MAOI 5-MeO-DMT is O-demethylated by CYP2D6 and becomes Bufotenine in the liver, but without a MAOI, it is mostly N-Demethylated by MAOI into 5-MeO-Indole-acetic-acid ([http://www.ncbi.nlm.nih.gov/pubmed/20206139 Shen et al 2010]; [http://www.springerlink.com/content/vk476215k424w65n/ Ai-Ming 2008). | + | When ingested together with a MAOI 5-MeO-DMT is O-demethylated by CYP2D6 and becomes Bufotenine in the liver, but without a MAOI, it is mostly N-Demethylated by MAOI into 5-MeO-Indole-acetic-acid ([http://www.ncbi.nlm.nih.gov/pubmed/20206139 Shen et al 2010]; [http://www.springerlink.com/content/vk476215k424w65n/ Ai-Ming 2008]). |
| − | There is | + | There is some concern regarding taking 5-MeO-DMT orally, specially with a MAOI. There has been at least one reported death (Sklerov, 2005; [http://www.google.com/url?sa=t&rct=j&q=5-meo-dmt%2Bayahuasca%2Bdeath&source=web&cd=7&ved=0CEcQFjAG&url=http%3A%2F%2Fbitnest.ca%2Fexternal.php%3Fid%3D%252506%25257C%252517%25250A6%252504D8EM%25255D%252527%252525%252523%25251B%252506WEW%25251CPIq&ei=qgO5Tp3qL4Wr8AOrzZCsBw&usg=AFQjCNGjI5n62EZtXjYKymYWEUb_YvisUA Callaway 2006]), and some hospitalizations. This might be due to individual metabolic differences. Check this thread for more info |
== Life-forms containing 5-MeO-DMT == | == Life-forms containing 5-MeO-DMT == | ||
Revision as of 11:30, 8 November 2011
| Note: | This page has been transcluded to The Nexian DMT Handbook under the 5-MeO-DMT section or other locations within or without the handbook. Please markup in consideration of this. The top section header is to remain in place as a reference for subsequent section headers and to allow easy editing directly from the handbook. |
Contents
- 1 Brief overview - What is 5-MeO-DMT?
- 2 Chemical and physical properties
- 3 Effects
- 4 Pharmacology, toxicity and general safety
- 5 Life-forms containing 5-MeO-DMT
- 6 Extraction teks
- 7 Dosages and consumption methods
- 8 History of usage
- 9 Analysis of 5-MeO-DMT
- 10 Scientific publications
- 11 Other links of interest
Brief overview - What is 5-MeO-DMT?
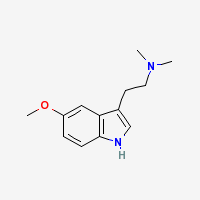
5-MeO-DMT is a potent naturally occuring psychedelic alkaloid.
Chemical and physical properties
For solubility, melting point, etc, check the 5-MeO-DMT Chemical and Physical Properties WIKI
Effects
Pharmacology, toxicity and general safety
When ingested together with a MAOI 5-MeO-DMT is O-demethylated by CYP2D6 and becomes Bufotenine in the liver, but without a MAOI, it is mostly N-Demethylated by MAOI into 5-MeO-Indole-acetic-acid (Shen et al 2010; Ai-Ming 2008).
There is some concern regarding taking 5-MeO-DMT orally, specially with a MAOI. There has been at least one reported death (Sklerov, 2005; Callaway 2006), and some hospitalizations. This might be due to individual metabolic differences. Check this thread for more info
Life-forms containing 5-MeO-DMT
Extraction teks
Any typical extraction teks for DMT should potentially extract 5-MeO-DMT (but it will also extract DMT together).
Dosages and consumption methods
5-MeO-DMT is around 5 times more potent than DMT by weight.
Smoked, dosage is around 5-15mg.
Oral, according to Jonathan Ott, 30mg are active without the need for MAOIs. Mixing with MAOIs, it will be 3 times stronger, so a dosage will be around 10mg. There is some concern about 5-MeO-DMT's oral ingestion safety, and it might be related with individual metabolism differencescheck this thread for more info. If you are consuming 5-MeO-DMT orally for the first time, specially with MAOIs, start VERY low and raise your dosage gradually.
Insufflated, 5-20mg (Erowid)
History of usage
Analysis of 5-MeO-DMT
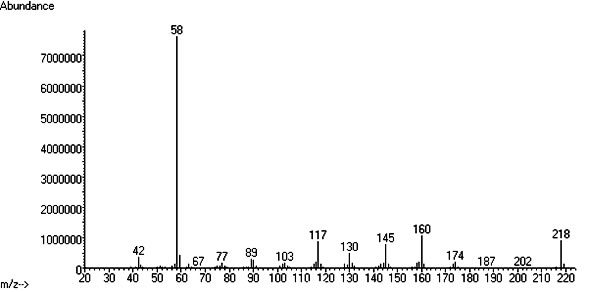
GC-MS
Retention time: 12.946 (System used)
Other info: 5-MeO-DMT: EI/MS (m/z, %): 218 (M+ , 10), 160 (6.3), 145 (2.5), 117 (5.0), 58 (100), 42 (4). (Takahashi 2008)
InfraRed
5-MeO-DMT freebase
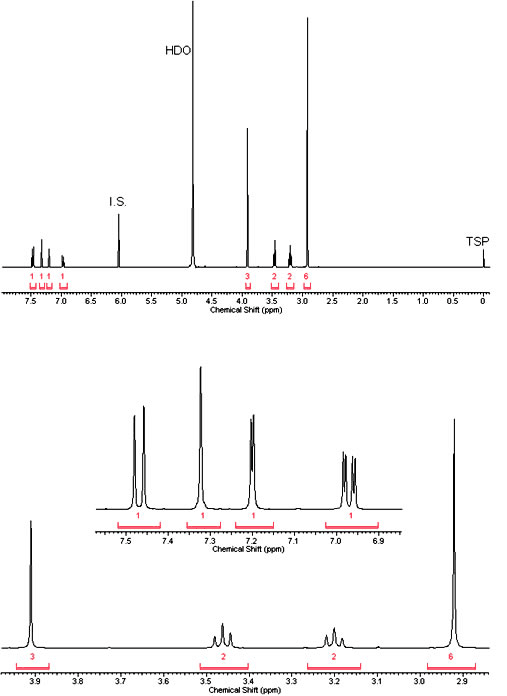
NMR
1H NMR (400 MHz, D2O) δ ppm 7.47 (d, J=8.90 Hz, 1 H) 7.32 (s, 1 H) 7.20 (d, J=2.45 Hz, 1 H) 6.97 (dd, J=8.90, 2.45 Hz, 1 H) 3.91 (s, 3 H) 3.46 (t, J=7.43 Hz, 2 H) 3.20 (t, J=7.43 Hz, 2 H) 2.92 (s, 6 H).
(Source: Microgram Bulletin Vol. 3, Pg 8)
Other info: 1 H-NMR (CDCl , δ): 2.34 (6 H, s), 2.63 (2 H, m), 2.91 (2 H, m), 3.86 (3 H, s), 6.85 (1 H, dd, J = 2.3, 8.6 Hz), 6.98 (1 H, br d, J = 2.3 Hz), 7.05 (1 H, d, J = 2.3 Hz), 7.22 (1 H, d, J = 8.6 Hz). 13 C-NMR (CDCl3 , δ): 23.7, 45.4, 56.0, 60.1, 100.8, 111.8, 112.1, 113.9, 122.3, 127.8, 131.5, 153.9. (Takahashi 2008)