Difference between revisions of "Conversion of Sodium Bicarbonate into Sodium Carbonate"
From DMT-Nexus Wiki
Endlessness (Talk | contribs) |
|||
| Line 7: | Line 7: | ||
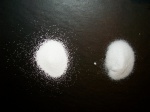
[[Image:Sodium carb vs bicarb.JPG|thumb|150px|Sodium carbonate on left and bicarbonate on right, both in oversaturated solutions.]] | [[Image:Sodium carb vs bicarb.JPG|thumb|150px|Sodium carbonate on left and bicarbonate on right, both in oversaturated solutions.]] | ||
[[Image:100_0180b.jpg|thumb|150px|left|After vs Before the conversion. Sodium carbonate on left and bicarbonate on right. Notice how carbonate is more grainy and bicarbonate more loose/fluffy ]] | [[Image:100_0180b.jpg|thumb|150px|left|After vs Before the conversion. Sodium carbonate on left and bicarbonate on right. Notice how carbonate is more grainy and bicarbonate more loose/fluffy ]] | ||
| − | # | + | # Weigh your sodium bicarbonate, and put it onto a non-aluminum pan or oven-safe dish. |
| − | # Place in the oven at 400ºF (205ºC) for one hour to release CO2. | + | # Place in the oven at 400ºF (205ºC) for one hour to one hour and a half to release CO2. |
| − | # The resulting material should have lost around third of the original weight. It will be of a slightly less powdery consistency, closer to sugar than flour. | + | # The resulting material should have lost around third of the original weight. It will be of a slightly less powdery consistency, closer to sugar than flour. If it didnt lose a third of the original weigh, leave it for longer in the oven |
#* ''sodium carbonate feels a bit looser and grainier than bicarbonate, and in an oversaturated solution, sodium bicarbonate will remain powdery while sodium carbonate tends to rock up.'' | #* ''sodium carbonate feels a bit looser and grainier than bicarbonate, and in an oversaturated solution, sodium bicarbonate will remain powdery while sodium carbonate tends to rock up.'' | ||
| − | {{ShowInfo/In Article|[[Image:Information.png]]|'''NOTE'''|This can also be done on a stove top/oven ring in a pot and take around | + | {{ShowInfo/In Article|[[Image:Information.png]]|'''NOTE'''|This can also be done on a stove top/oven ring in a pot and take around 10 minutes to completely dehydrate |
|0px | |0px | ||
| | | | ||
Revision as of 13:46, 13 May 2012
| Note: | This page has been transcluded to The Nexian DMT Handbook under the Conversion of Sodium Bicarbonate into Sodium Carbonate section or other locations within or without the handbook. Please markup in consideration of this. The top section header is to remain in place as a reference for subsequent section headers and to allow easy editing directly from the handbook. |