Difference between revisions of "CIELO"
(→Introduction 🙏) |
(Large update to jar crystallization and other better methods) |
||
| Line 3: | Line 3: | ||
| − | In this technique (TEK), | + | In this technique (TEK), cold alkaline cactus paste (Fig. 2) is extracted with chilled ethyl acetate (Fig. 3). Mescaline citrate is precipitated with citric acid (Fig. 4) and collected (Fig. 5). |
| Line 15: | Line 15: | ||
= Materials 🛒= | = Materials 🛒= | ||
| − | * | + | *French press (or similar ensemble) |
| − | + | *300g ice cold water | |
| − | + | *25g lime | |
| − | + | *100g dry cactus powder | |
| − | + | *1qt ethyl acetate (also sold as "MEK substitute") | |
| − | * | + | *Coffee filter, support basket, and funnel |
| − | * | + | *Quart jar |
| − | * | + | *4g of citric acid |
| − | * | + | *Washing soda (for solvent reclaim) |
| − | * | + | |
| − | + | ||
| − | + | [[File:IMG 20210608 223040865 copy 800x600.jpg|center]] | |
| − | + | <center>''Fig. 1: Materials.</center> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | [[File:IMG | + | |
| − | <center>''Fig. 1: Materials | + | |
= Process 📜= | = Process 📜= | ||
| − | == | + | == Paste 🌵 == |
| − | Mix water and | + | Mix ice cold water, lime, and cactus in french press for a few minutes to a smooth paste (Fig. 2). |
[[File:IMG 20210603 183405358 copy 800x600.jpg|center]] | [[File:IMG 20210603 183405358 copy 800x600.jpg|center]] | ||
| − | <center>''Fig. 2: | + | <center>''Fig. 2: Alkaline cactus paste.</center> |
| − | == | + | == Extract 👨🏾🔬== |
| − | + | Cover cold paste with chilled ethyl acetate (~0F), mix for for 60 seconds and filter to quart jar. Repeat until quart jar is ~80% full (~5x). | |
| − | Inspect extract for droplets or particles. If present, | + | Inspect extract for droplets or particles. If present, remove them. '''Extract needs to be clean''' (see Fig. 3). |
| − | '''Extract needs to be clean''' (see Fig. 3). | + | |
| Line 60: | Line 49: | ||
<center>''Fig. 3: Ethyl acetate extract.</center> | <center>''Fig. 3: Ethyl acetate extract.</center> | ||
| − | == Crystalize | + | == Crystalize ✨== |
| − | + | Drop (do not stir) citric acid into extract. Clouds quickly form first followed after a few hours by mescaline citrate crystals (Fig. 4). Allow crystalization to complete undisturbed (~12 hours). | |
| − | [[File:IMG | + | [[File:IMG 20210609 064813073 copy 800x600 1.jpg|center]]<center>''Fig. 4: Crystals in ethyl acetate.</center> |
| − | <center>''Fig. 4: | + | |
| + | == Collect 💖== | ||
| + | Catch crystals in a coffee filter. Rinse jar/filter with room temperature ethyl acetate a couple times and dry (Fig. 5). | ||
| − | |||
| + | If some crystals stubbornly remain on the jar walls, dissolve them in hot water, dry in a shallow dish, and scrape. | ||
| − | |||
| − | |||
| − | + | Yield depends on the cactus and is usually between 0.2% to 2% with ~1% being common<ref>Cactus analysis thread[https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=71353]</ref>. | |
| − | + | ||
[[File:IMG 20210603 130102387 copy 600x800 copy 800x600.jpg|center]] | [[File:IMG 20210603 130102387 copy 600x800 copy 800x600.jpg|center]] | ||
| − | <center>''Fig. | + | <center>''Fig. 5: Collected mescaline citrate crystals.</center> |
| − | Mass spectrometry (MS) results from solaris analytical<ref>Solaris analytical service[https://www.solarisanalytical.com/]</ref> indicate the product is very clean mescaline (Fig. | + | Mass spectrometry (MS) results from solaris analytical<ref>Solaris analytical service[https://www.solarisanalytical.com/]</ref> indicate the product is very clean mescaline (Fig. 6). |
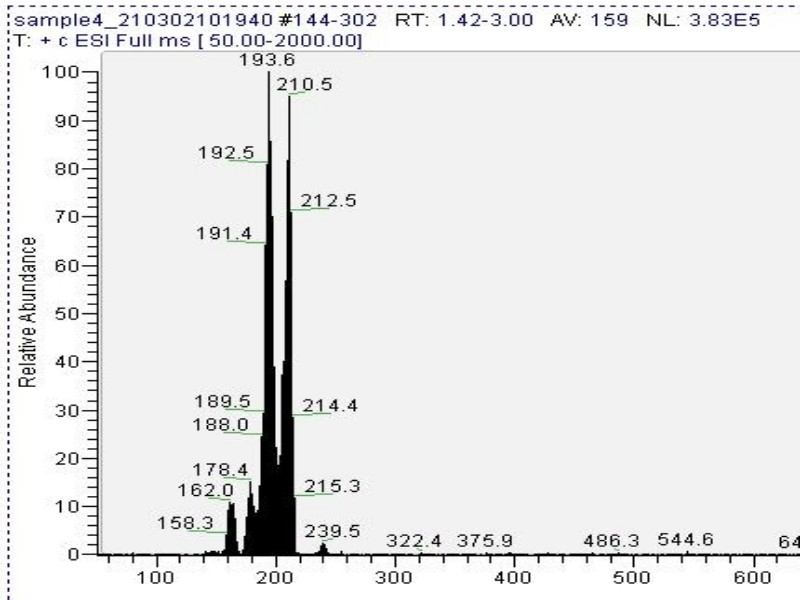
| − | [[File: Cactus-extract copy 800x600_1.jpg|center]]''<center>Fig. | + | [[File: Cactus-extract copy 800x600_1.jpg|center]]''<center>Fig. 6: Mass spectrometry result. Peak near 210.5 is mescaline. Lower mass peaks are mescaline with cleaved functional groups. The peak at 239.5 is not attributed to mescaline. </center> |
== Reclaim Solvent 💚== | == Reclaim Solvent 💚== | ||
| Line 91: | Line 78: | ||
| − | Wash spent extract with sodium carbonate saturated water shaking vigorously (emulsions do not form). Citric acid removal | + | Wash spent extract with sodium carbonate saturated water shaking vigorously (emulsions do not form). Citric acid removal is complete when CO2 bubbling stops. Filter any excess calcium carbonate and remove water layer. Freeze and filter out ice crystals. Store for reuse. |
= Appendix: Development Notes 🔬= | = Appendix: Development Notes 🔬= | ||
== Paste 🌵== | == Paste 🌵== | ||
| − | No improvements were seen with longer basing time, microwaving, | + | No improvements were seen with longer basing time, microwaving, drying, or increasing the ionic strength. |
| − | == | + | == Extract 👨🏾🔬== |
| − | + | Longer and/or warmer pulls resulted in darker color, smaller crystals and more absorbed solvent with no yield benefit. | |
| − | + | Chemically drying the extract before salting had no crystallization or yield benefit. | |
| − | == | + | == Crystalize ✨== |
| − | + | During crystallization, every 233mg of citric acid ('''H3Cit''') react with free base mescaline ('''Mes''') to form to 1g of mescaline citrate (or slightly more if a hydrate is precipitating): | |
| − | + | '''<span style="color: Orange"> <div style="text-align: center;">3Mes<sub>(↑)</sub> + H3Cit<sub>(↑)</sub> ⇒ 3(MesH)Cit<sub>(↓)</sub></div></span>''' | |
| − | |||
| − | |||
| + | Excess citric acid shifts the precipitation reaction to the right (Le Chatelier's principle), helping overcome water and plant material. There is a lot room for excess citric acid in solution since its solubility is 50mg/g in ethyl acetate. The TEK recommends ~5mg/g but since cacti and pull techniques can vary, users may find other values work better for their specific situation (in one example with whole cactus powder 20mg/g was used <ref>Ethyl acetate approach[https://www.dmt-nexus.me/forum/default.aspx?g=posts&t=96262]</ref>). | ||
| − | |||
| + | Several factors can make crystals smaller: Reusing ethyl acetate, warmer pulls, longer pulls, higher citric acid concentration, mechanical agitation, and other potential variables. Small crystals can look like a fine powder. Potency does not seem affected by the crystallization appearance, and a powdery precipitate is not a problem unless it becomes difficult to decant/filter. | ||
| − | |||
| + | After the initial crystallization, adding more citric acid and/or moving the extract to the refrigerator did not result in any more precipitation. Moving the extract to the freezer produced ice crystals. | ||
| − | |||
| + | Other dry organic acids could work. Fumaric, Malic, Tartaric, Ascorbic, Succinic, etc can be tested in future investigations. | ||
| − | + | == Collect 💖== | |
| − | + | Washing crystals over a filter gave better results than decanting. The filter paper wicked away plant off colors forming a ring. Unlike the pulls, warmer ethyl acetate is an advantage because the goal is to dissolve away plant matter. | |
| + | |||
| − | + | The jar wash should be done immediately. If any straggler crystals dry in the jar they stuck to the wall. | |
| − | + | ||
= References 🗝️= | = References 🗝️= | ||
<references/> | <references/> | ||
Revision as of 13:00, 9 June 2021
Contents
Introduction 🙏
CIELO stands for Crystals In Ethyl-acetate Leisurely Over-the-counter.
In this technique (TEK), cold alkaline cactus paste (Fig. 2) is extracted with chilled ethyl acetate (Fig. 3). Mescaline citrate is precipitated with citric acid (Fig. 4) and collected (Fig. 5).
Thanks to everyone who contributed: someblackguy, Benzyme, shroombee, Metta-Morpheus, Downwardsfromzero, Kash, grollum, Mindlusion, Doubledog, Dreamer042, Loveall, and others.
Safety ⛑️
Review ethyl acetate's safety information[1]. Verify MSDS, plastic compatibility, and clean evaporation.
Following this advice does not guarantee safety. It is up to each adult individual to make their own decision.
Materials 🛒
- French press (or similar ensemble)
- 300g ice cold water
- 25g lime
- 100g dry cactus powder
- 1qt ethyl acetate (also sold as "MEK substitute")
- Coffee filter, support basket, and funnel
- Quart jar
- 4g of citric acid
- Washing soda (for solvent reclaim)
Process 📜
Paste 🌵
Mix ice cold water, lime, and cactus in french press for a few minutes to a smooth paste (Fig. 2).
Extract 👨🏾🔬
Cover cold paste with chilled ethyl acetate (~0F), mix for for 60 seconds and filter to quart jar. Repeat until quart jar is ~80% full (~5x).
Inspect extract for droplets or particles. If present, remove them. Extract needs to be clean (see Fig. 3).
Crystalize ✨
Drop (do not stir) citric acid into extract. Clouds quickly form first followed after a few hours by mescaline citrate crystals (Fig. 4). Allow crystalization to complete undisturbed (~12 hours).
Collect 💖
Catch crystals in a coffee filter. Rinse jar/filter with room temperature ethyl acetate a couple times and dry (Fig. 5).
If some crystals stubbornly remain on the jar walls, dissolve them in hot water, dry in a shallow dish, and scrape.
Yield depends on the cactus and is usually between 0.2% to 2% with ~1% being common[2].
Mass spectrometry (MS) results from solaris analytical[3] indicate the product is very clean mescaline (Fig. 6).
Reclaim Solvent 💚
Reusing solvents is encouraged[4] at the DMT nexus.
Wash spent extract with sodium carbonate saturated water shaking vigorously (emulsions do not form). Citric acid removal is complete when CO2 bubbling stops. Filter any excess calcium carbonate and remove water layer. Freeze and filter out ice crystals. Store for reuse.
Appendix: Development Notes 🔬
Paste 🌵
No improvements were seen with longer basing time, microwaving, drying, or increasing the ionic strength.
Extract 👨🏾🔬
Longer and/or warmer pulls resulted in darker color, smaller crystals and more absorbed solvent with no yield benefit.
Chemically drying the extract before salting had no crystallization or yield benefit.
Crystalize ✨
During crystallization, every 233mg of citric acid (H3Cit) react with free base mescaline (Mes) to form to 1g of mescaline citrate (or slightly more if a hydrate is precipitating):
Excess citric acid shifts the precipitation reaction to the right (Le Chatelier's principle), helping overcome water and plant material. There is a lot room for excess citric acid in solution since its solubility is 50mg/g in ethyl acetate. The TEK recommends ~5mg/g but since cacti and pull techniques can vary, users may find other values work better for their specific situation (in one example with whole cactus powder 20mg/g was used [5]).
Several factors can make crystals smaller: Reusing ethyl acetate, warmer pulls, longer pulls, higher citric acid concentration, mechanical agitation, and other potential variables. Small crystals can look like a fine powder. Potency does not seem affected by the crystallization appearance, and a powdery precipitate is not a problem unless it becomes difficult to decant/filter.
After the initial crystallization, adding more citric acid and/or moving the extract to the refrigerator did not result in any more precipitation. Moving the extract to the freezer produced ice crystals.
Other dry organic acids could work. Fumaric, Malic, Tartaric, Ascorbic, Succinic, etc can be tested in future investigations.
Collect 💖
Washing crystals over a filter gave better results than decanting. The filter paper wicked away plant off colors forming a ring. Unlike the pulls, warmer ethyl acetate is an advantage because the goal is to dissolve away plant matter.
The jar wash should be done immediately. If any straggler crystals dry in the jar they stuck to the wall.