Difference between revisions of "CIELO"
| Line 1: | Line 1: | ||
| − | {{ShowInfo|[[Image:Note_error.png]]|'''Note:'''|This page is a workplace for an experimental process. It is intended to summarize information from ongoing discussions in the forum. Feel free to update this page with any observations or comments. Wiki changes are reversible so you can't break anything - be bold. This page is not currently linked to the main wiki and will remain as such until the process is independently verified by others.} | + | {{ShowInfo|[[Image:Note_error.png]]|'''Note:'''|This page is a workplace for an experimental process. It is intended to summarize information from ongoing discussions in the forum. Feel free to update this page with any observations or comments. Wiki changes are reversible so you can't break anything - be bold. This page is not currently linked to the main wiki and will remain as such until the process is independently verified by others.} |
| − | + | ||
= Introduction = | = Introduction = | ||
Revision as of 15:46, 18 March 2021
{{ShowInfo|![]() |Note:|This page is a workplace for an experimental process. It is intended to summarize information from ongoing discussions in the forum. Feel free to update this page with any observations or comments. Wiki changes are reversible so you can't break anything - be bold. This page is not currently linked to the main wiki and will remain as such until the process is independently verified by others.}
|Note:|This page is a workplace for an experimental process. It is intended to summarize information from ongoing discussions in the forum. Feel free to update this page with any observations or comments. Wiki changes are reversible so you can't break anything - be bold. This page is not currently linked to the main wiki and will remain as such until the process is independently verified by others.}
Contents
Introduction
CIELO stands for Crystals In Ethyl-acetate Leisurely OTC (Over The Counter).
In this TEK, aqueous alkaline cactus paste is extracted with ethyl acetate. The extract is salted with citric acid to precipitate mescaline citrate crystals directly in the solvent.
This process was developed in a loving collaborarion at the DMT nexus. Deep thanks and gratitude to everyone who contributed. Sharing this information can be done if left unchanged and this source is referenced. This process may never be used for profit and is declared here as freely disclosed art that cannot be patented.
Safety
Review ethyl acetate's safety information[1] and check the manufacture's MSDS to verify you have pure ethyl acetate.
Make sure any plastic you use is compatible with ethyl acetate. Verify your ethyl acetate evaporates cleanly leaving no residue.
Each adult individual needs to find and review any other relevant safety information throughly and make their own personal decision on proceeding.
Materials
- Quart jars
- 300g water
- 25g Ca(OH)2 (lime)
- 100g powdered dry cacti
- ~ 1000g ethyl acetate ("MEK substitute")
- Citric acid
- Coffee filter
Process
Paste
Mix water and lime. Add cactus powder and mix/rest/mix to a homogeneous smooth paste. Total lime reaction time should be at least one hour.
Pull
Add ~ 250g of ethyl acetate to the paste and mix gently for 30 seconds. Rest overnight (paste will become compact) and decant to a second jar.
It is important to never shake or stir quickly to minimize the ammount of solvent binding to the paste.
Pull three more times with 175g of ethyl acetate.
Combined pulls will give ~675g (750ml) of deep green extract (~100g will be absorbed during the first pull). Image below shows the finshed extract against a window with horizontal blinds.
Salt
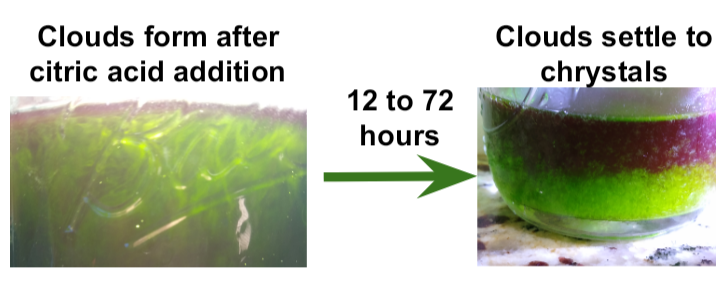
Dissolve ~ 3.5g (~1/2 tsp) of citric acid into the extract making it cloudy.
Move extract to fridge for at least 72h. White xtals will form at the bottom of the jar and a smaller amount may stick to the jar walls.
Finish
Shake precipitated extract to loosen up any crystals on the jar walls. Pour through a coffee filter to catch the crystals.
Shake jar with fresh ethyl acetate to knock off any wall crystals that remain and pour that trough the filter. Repeat a few times until green color is wicked away (give time to allow some wicking to occur between rinses). Let dry, this is the final product.
If a small amount of crystals stubbornly stick to the jar walls they can be recovered by dissolving in water and evaporating. This results in long needles and can also be done for the main product that was recovered in the coffee filter.
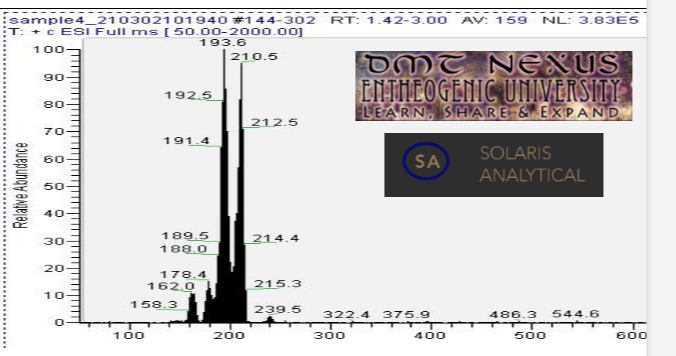
Mass spectrometry (MS) results from solaris analytical[2] indicate the product is very clean mescaline. See MS spectrum below, peak near 210.5 is mescaline. Peaks at and 193.6, 178.4, and 162.0 are believed to be mescaline with amine/methyl/methoxy groups cleaved to generate the lower mass mescaline spectrum in multiples of ~16 au (16.9, 32.1, and 48.5 respectively). The small peak at 239.5 is not attributed to mescaline.
Lab Notes
The pulls appear to be very efficient, extracting mescaline within minutes. The similarity between the solvent's ethoxy group and mescaline's methoxy groups could contribute to the solubility. The long soak is added to the tek to make the paste compact and trivial to decant.
It is possible to chemically dry the extract with a drying agent such as anhydrous MgSO4 before salting. However, no clear yield benefit was observed by performing this step. Surprisingly, solutions carefully dried with anhydrous CaCl2 followed by K2CO3 had difficulty crystalizing, indicating that a some water is desirable for crystalization. This points to the precipitate from this TEK maybe being hydrate salt. Regardless of the reason, the water content in ethyl acetate directly from the pulls is in a good range experimentally.
During salting, every 10mg of citric acid (CitH3) reacts with enough free base mescaline (Mes) to precipitate up to 43mg of mescaline citrate (or slightly more if a hydrate form is precipitating):
250mg of citric acid are enough to convert mescaline from free base to salt for the typical cactus (up to 1% yield). However, and outlier like the legendary Ogun would need ~1100mg of citric acid for a 4.7% yield.
After neutralizing, over acidifying is usually necessary to induce crystalization. This is a simple but very important lab observation, compatible with Le Chatelier's principle. There is room for excess citric acid in solution since several grams can dissolve in a quart of ethyl acetate even when chilled. The vast majority of excess acid is poured off after salting, and any traces removed when washing the crystals.
Other solid organic acids could work. Fumaric, Malic, Tartaric, Ascorbic, Succinic, etc can be tested. Sulfuric acid HCl could be looked at (and crystals have been observed with sulfuric), but may interact with ethyl acetate.
It is possible that some of the assumptions and conclusions in these lab notes are incorrect or incomplete. The process was tested in several ways, but the search was not exhaustive[3]. There could be ways to improve this process.
References
Appendix: Shroombee's Notes
Experiment #2
March 12, 2021
8:45 am Weighed out 100 grams cactus chips (will use 50 grams for this experiment and the other 50 grams in a followup experiment).
8:48 am Ground cactus chips to very fine powder in Vitamix for 2 minutes, eventually hitting highest speed.
8:50 am Weighed out 12.5 grams lime, 153.1 grams purified water, 50.5 grams finely ground cactus powder.
8:58 am Mixed water and lime together in a stainless steel mixing bowl to make a milky water. Gradually added cactus powder, incorporating powder into milky water. Cactus gets to a fluffy texture and then transitions to being a little more clumpy. Finished mixing at 9:05 am.
9:11 am Added 99.8 grams ethyl acetate to the cactus for pull #1. Mixed gently for 30 seconds.
In retrospect, do not mix at all. Just push the cactus around a little so the ethyl acetate can run through the cactus. Solvent quickly turns green.
9:23 am After letting the cactus and ethyl acetate sit for 10 minutes, decanted the ethyl acetate into a beaker with a metal coffee filter sitting inside the top of the beaker (works well). Beaker plus filter beforehand was 342.3 grams. After decanting solvent into it, 372.8 grams.
Solvent recovery for pull #1 = 30.5 grams (out of 99.8 grams added). For next experiment, see if not mixing at all will reduce the amount of solvent that the cactus absorbs. A drop of solvent on pH paper turns the paper dark green (not blackish green though).
9:28 am Added 110.1 grams fresh ethyl acetate to cactus for pull #2. Very gently pushing the cactus for about 10 seconds, just to get the solvent to run through the cactus. The ethyl acetate quickly turns green.
9:31 am Moved pull #1 to a mason jar. Mason jar beforehand is 426.8 grams. After is 456.6 grams. 29.8 grams solvent moved from beaker to mason jar.
9:38 am After 10 minutes sitting with the cactus, decanted pull #2 into the beaker. Beaker is 342.3 grams before, 447.9 grams after.
Solvent recovery for pull #2 = 105.6 grams (out of 110.1 grams added). pH paper is medium dark green.
9:45 am Added 103.3 grams fresh ethyl acetate to the cactus for pull #3. Almost no manipulation of the cactus at this point. Solvent quickly gets green color.
9:46 am Moved pull #2 to the mason jar (combining with pull #1). Mason jar beforehand is 456.4 grams. After is 560.4 grams. 104.0 grams solvent moved from beaker to mason jar. Interesting at 9:31 am the mason jar weighed 456.6 grams. 15 minutes later it weighs 456.4 grams. Is this evaporation of ethyl acetate or error in the scale? Scale supposed to be accurate to 0.1 grams.
9:55 am After 10 minutes sitting with the cactus, decanted pull #3 into the beaker. Beaker is 342.5 grams before, 450.3 grams after.
Solvent recovery for pull #3 = 107.8 grams (out of 103.3 grams added). We got back the amount we added for pull #3, plus a few more grams. The paste is starting to stick to itself, making it easy to decant and pour solvent out of the mixing bowl by holding the paste back with a strainer/skimmer ladle. pH paper is light green.
10:00 am Added 103.9 grams fresh ethyl acetate to the cactus for pull #4. As with pull #3, almost no manipulation of the cactus. Just pushing the cactus under the solvent. Solvent picks up green color.
10:08 am Moved pull #3 to the mason jar (combining with pull #1 and pull #2). Mason jar beforehand is 560.2. After is 667.0 grams. 106.8 grams solvent moved from beaker to mason jar. As before, mason jar seems to have lost 0.2 grams of solvent. The solvent in the mason jar is a beautiful emerald green.
10:10 am After 10 minutes sitting with the cactus, decanted pull #4 into the beaker. Beaker is 342.7 grams before, 444.7 grams after.
Solvent recovery for pull #4 = 102.0 grams (out of 103.9 grams added). pH paper is faint green. After drying, the paper barely has any greenish tint.
10:16 am Added 104.0 grams fresh ethyl acetate to the cactus for pull #5. Almost no manipulation of the cactus. Solvent picks up a light green tint.
10:20 am Moved pull #4 to the mason jar (combining with pull #1-3). Mason jar beforehand is 666.9 grams. After is 767.8 grams. 100.9 grams solvent moved from beaker to mason jar. Mason jar lost 0.1 grams solvent since 10:08 am.
10:26 am After 10 minutes sitting with the cactus, decanted pull #5 into the beaker. Beaker is 342.5 grams before, 446.0 grams after.
Solvent recovery for pull #5 = 103.5 grams (out of 104.0 grams added). pH paper has no materially significant color change. After drying, the paper shows no color change. This pull is NOT being combined with pulls #1-4 since it does not appear to have any freebase mescaline. Will consider what technique to use to see if there is any mescaline to be obtained from this pull.
Total solvent added = 99.8 + 110.1 + 103.3 + 103.9 + 104.0 = 521.1 grams. Total solvent recovered = 30.5 + 105.6 + 107.8 + 102.0 + 103.5 = 449.4 grams. Lost 71.7 grams solvent to the cactus (almost all in pull #1).
10:46 am Washed and dried the metal coffee filter. Then added a paper filter into the metal filter. Ran pulls #1-4 through the metal+paper filter and into a new mason jar. Ran pull #5 through the metal+paper filter and into a separate mason jar. The purpose of filtering again is that I noticed a few bits of cactus material got into the combined decanted solvent. I don't know how that occurred since the metal filter is a fine mesh filter. In any case, now we have filtered solvent.
10:52 am Added 254 grams of citric acid to the combined pulls mason jar. Clouds form in the solvent. Stirred gently with a spoon. pH paper comes out yellow.
10:56 am Put a stainless steel lid with silicone liner on the mason jar. Put mason jar into the frig to wait for crystals.
1:45 pm Mason jar is in the frig. Solvent is opaque. No crystals yet.
Added by Loveall: This looks promising. If xtals do not form, I would try adding citric acid until pH paper has some red coloring (maybe another 250mg will do). If xtals still don't form, I would add water at ~0.5% increments (~2.2g), shaking and waiting to see if xtals form. I added a picture of how the pH paper looks from an extract that crystalized well in the main TEK.
Experiment #1
March 9, 2021
6:42 pm
100 grams Peruvian Torch chips
298 grams purified water
Blended cactus chips in the Vitamix dry container so it becomes a fine powder.
6:58 pm Mixed cactus powder and water in a plastic mixing bowl rather than a mason jar because it seems easier. Product is a fluffy texture, olive green color. There is no excess water.
The bowl plus cactus plus silicone mixing spoon weighs 826.5 grams. Microwave on high for 30 seconds, mix, then weigh. This is pretty easy. I wouldn't want to do this in a mason jar. The cactus does not bubble and there is no issue at 30 seconds with anything bubbling over. Weight after each 30 second cycle:
825.6 grams
823.9 grams
820.2 grams
816.0 grams
808.7 grams
800.7 grams
791.3 grams
783.2 grams
775.8 grams
765.8 grams
DONE
After a couple of the microwaving cycles, the cactus lost its fluffiness and became like light bread dough, not sticking much to the bowl.
7:25 pm Begin slowly mixing in 25 grams pickling lime. Started with the plastic bowl but switched to a ceramic bowl after a minute, not knowing how the plastic would react to the base. Bowl appears fine after washing it out.
7:39 pm I'm using a fork to try to get the lime mixed evenly. I figure my Kitchenaid stand mixer will be easier so I break that out.
7:45 pm Letting the Kitchenaid stir the cactus for me. Much easier!
7:53 pm Added 25 grams calcium chloride. Before adding, the cactus paste was fluffy and stuck to the sides of the stainless steel bowl. After adding the calcium chloride, the cactus became like clumpy sand and hardly stuck to the sides of the bowl.
7:58 pm Stopped mixing.
8:05 pm Transferred cactus to a wide mouth quart mason jar and added 220 ml of ethyl acetate (weighing 199.9 grams). The ethyl acetate came up to approximately 540 ml on the side of the mason jar. After shaking, the cactus looked like fluffy beach sand.
8:28 pm Shake for a minute.
9:00 pm Shake for a minute.
9:22 pm No shaking, I notice the ethyl acetate is light green.
10:30 pm Shake for a minute. The cactus is starting to get a little stickier, leaving streaks on the glass.
11:00 pm Ethyl acetate comes up to exactly 500 ml on the side of the mason jar. Versus ~540 ml at 8:05 pm when the ethyl acetate was first added. What accounts for this 40 ml difference?
12:00 am Shake for a minute.
1:06 am Weighed mason jar with cactus: 1001.4 grams.
March 10, 2021
7:57 am Weighed mason jar with cactus: 1001.1 grams.
Ready to decant ethyl acetate. Large pyrex beaker with metal coffee filter weighs 342.3 grams. Ethyl acetate is a medium, emerald green. Definitely not light green and not yellow.
Using a small ladle to decant, which gets most of the ethyl acetate with no plant matter. Beaker weighs 427.2 grams meaning we recovered 84.9 grams of ethyl acetate. Not too good.
I transferred the cactus to a french press to see if I could recover more ethyl acetate. The sticky paste does not compress and I recovered an additional ~6 grams ethyl acetate. French press is obviously not worth the effort!
8:21 am Done with decanting, transferring, french pressing, weighing, et cetera. Total recovery for this pull is 90.6 grams ethyl acetate.
8:23 am Added 90.7 grams of fresh ethyl acetate to the mason jar. Stirred ethyl acetate into the sticky cactus, did not shake. Ethyl acetate is already changing to green color.
8:33 am Photo of pH paper. Paper is dark green. pH 10 or 11? Also tested using the 4 color pH strips. More difficult to judge the pH with these.
8:34 am Photo of recovered ethyl acetate showing it is an emerald green color.
8:45 am Poured 10 ml of ethyl acetate into a small beaker. Added 13 mg of citric acid. Lower half of the beaker turned milky, cloudy. Checked pH and it's basically the same as the rest of the solvent. So the small amount of citric acid didn't change the pH much. I used a disposable pipette and pulled from the top of the solvent, so perhaps the top layer didn't have a chance to react yet.
8:49 am Photo showing cloudy 10 ml in small beaker.
8:50 am Poured the 10 ml and the rest of the solvent into a mini Pyrex baking dish, using the larger volume of solvent to rinse out of the small beaker. I probably should have left the solvent in a mason jar or beaker rather than adding to the baking dish. Added 95 mg of citric acid. Stirred a little because I wanted to get a more accurate pH. pH was about 7. Added an additional 61 mg citric acid. pH went to about 6. Total citric acid added to the 90.6 grams ethyl acetate is 169 mg. Even after rinsing with the main solvent, the bottom of the small beaker has some spots that look like something crystalizing. I don't know if it's just sticky citric acid or ???
9:07 am Photo of 3 pH test strips.
3:42 pm Moving the dish to the freezer. Solvent is clear (and has been clear for at least a few hours). Initially I thought there were droplets on the bottom of the dish (around 11:00 am). Then tried to scrape at them with a knife and not seeing any movement, I figured they were a reflection from the surface because I noticed some tiny droplets on the surface if I caught the light at the right angle. Then as I tipped the dish at 3:42pm to move it into the freezer, I now see the droplets really are at the bottom of the dish. Sort of an oily substance. There are no crystals.
8:00 pm Retrieving pull #2. Beaker plus metal coffee filter weigh 342.3 grams. Weighs 407.5 grams after decanting. Recovered 65.2 grams. Looks like the cactus sucked up more solvent even though I didn't shake. pH is basic, although the pH paper is not as dark green as pull #1.
8:09 pm Removed a few tablespoons of sticky slimy cactus and put it into a separate bowl. Mixed in 5.4 grams of calcium chloride (which is a lot relative to the amount of cactus). Even waiting more than 30 minutes, no additional solvent is released. The cactus is drier though, more clumpy, and lost some gooey sliminess.
8:27 pm Since only 65.2 grams of solvent was retrieved, I decided adding this amount of fresh solvent for pull #3 would be too inefficient. I added 197.6 grams fresh ethyl acetate and stirred for 1-2 minutes. Solvent turned a light green fairly quickly.
8:38 pm Added 49 mg citric acid to the recovered solvent. Bottom of jar got milky. Tested pH with 3 strips. (1) Took a solvent sample towards the bottom of the jar at the clouds: pH about 5. (2) Solvent sample at the surface: pH about 7. (3) Swirled the jar around then took a solvent sample. pH about 6.
8:45 pm Put jar into freezer.