Difference between revisions of "Conversion of Sodium Bicarbonate into Sodium Carbonate"
From DMT-Nexus Wiki
(created page for handbook transclusion) |
Endlessness (Talk | contribs) |
||
| (18 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| + | <noinclude>{{Handbook Transclusion Header}}</noinclude> | ||
| + | |||
<onlyinclude> | <onlyinclude> | ||
{{procedure | {{procedure | ||
|[[Conversion of Sodium Bicarbonate into Sodium Carbonate]] | |[[Conversion of Sodium Bicarbonate into Sodium Carbonate]] | ||
| | | | ||
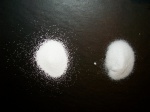
| − | # | + | [[Image:Sodium carb vs bicarb.JPG|thumb|150px|Sodium carbonate on left and bicarbonate on right, both in oversaturated solutions.]] |
| − | # Place in the oven at 400ºF for one hour | + | [[Image:100_0180b.jpg|thumb|150px|left|After vs Before the conversion. Sodium carbonate on left and bicarbonate on right. Notice how carbonate is more grainy and bicarbonate more loose/fluffy ]] |
| − | # | + | # Weigh your sodium bicarbonate, and put it onto a non-aluminum pan or oven-safe dish. |
| − | {{ShowInfo/In Article|[[Image:Information.png]]|'''NOTE'''|This can also be done on a stove top/oven ring in a pot and take around | + | # Place in the oven at 400ºF (205ºC) for one hour to one hour and a half to release CO2 and water. Alternatively you can put in a stainless steel (dont use any other material!) pot on the stovetop, 20mins should be enough. Be careful because the powder will be VERY hot, leave it to cool down for a while. |
| + | # The resulting material should have lost around 20% of the original weight. It will be of a slightly less powdery consistency, closer to sugar than flour. If it didnt lose a third of the original weigh, leave it for longer in the oven | ||
| + | #* ''sodium carbonate feels a bit looser and grainier than bicarbonate, and in an oversaturated solution, sodium bicarbonate will remain powdery while sodium carbonate tends to rock up.'' | ||
| + | {{ShowInfo/In Article|[[Image:Information.png]]|'''NOTE'''|This can also be done on a stove top/oven ring in a pot and take around 10 minutes to completely dehydrate | ||
|0px | |0px | ||
| | | | ||
Latest revision as of 12:31, 26 August 2013
| Note: | This page has been transcluded to The Nexian DMT Handbook under the Conversion of Sodium Bicarbonate into Sodium Carbonate section or other locations within or without the handbook. Please markup in consideration of this. The top section header is to remain in place as a reference for subsequent section headers and to allow easy editing directly from the handbook. |