Difference between revisions of "Psychedelic Compounds Chemical and Physical Properties"
(→DMT Citrate) |
(→Freebase DMT) |
||
| (19 intermediate revisions by 2 users not shown) | |||
| Line 31: | Line 31: | ||
Very Soluble in Xylene, Toluene, Limonene, acetone, Isopropyl Alcohol (IPA), methanol, ethanol, Dichloromethane (DCM), chloroform, ether, Butanone (also known as methyl ethyl ketone (MEK)) and butanol. | Very Soluble in Xylene, Toluene, Limonene, acetone, Isopropyl Alcohol (IPA), methanol, ethanol, Dichloromethane (DCM), chloroform, ether, Butanone (also known as methyl ethyl ketone (MEK)) and butanol. | ||
| − | Soluble in naphtha, hexane, heptane but almost insoluble in these solvents at freezing temperatures | + | Soluble in naphtha, hexane, heptane but almost insoluble in these solvents at freezing temperatures. Following daya from [https://www.dmt-nexus.me/forum/default.aspx?g=posts&m=1090269#post1090269 Source: Brennendes Wasser] |
| + | |||
| + | *Naphtha (80 °C, C6-C7, 60-80 °C) "infinite" (the DMT will melt and just create a homogeneous liquid-liquid solution. In the end it may even be a solution of Naphtha in DMT) | ||
| + | *Naphtha (20 °C, C6-C7) 2,93 g in 100 ml | ||
| + | *Naphtha in the freezer (-20 °C, C6-C7) 109 mg in 100 ml | ||
| + | *n-Hexane (20 °C) 770 mg in 100 ml | ||
| + | *n-Hexane in the freezer (-20 °C) 83 mg in 100 ml | ||
| + | *n-Pentane in the freezer (-20 °C) 50 mg in 100 ml | ||
| + | *n-Heptane in the freezer (-20 °C) 92 mg in 100 ml | ||
| + | |||
Almost insoluble in water. | Almost insoluble in water. | ||
| Line 76: | Line 85: | ||
==== DMT Benzoate ==== | ==== DMT Benzoate ==== | ||
| + | [[File:DMT-benzoate_3d_model.gif]] | ||
| + | |||
* Appearance: Crystal | * Appearance: Crystal | ||
* Solubility: Soluble in water and ethanol | * Solubility: Soluble in water and ethanol | ||
| + | * Mostly soluble in Isopropyl alcohol (at room temperature) | ||
* Poorly soluble in room temp propylene glycol | * Poorly soluble in room temp propylene glycol | ||
| Line 83: | Line 95: | ||
*Appears to not form in ethyl acetate but is mostly insoluble in ethyl acetate? (at room temperature.) | *Appears to not form in ethyl acetate but is mostly insoluble in ethyl acetate? (at room temperature.) | ||
| − | *Appears to be mostly insoluble in Acetone | + | *Appears to be mostly insoluble in Acetone and MEK at room temperature. |
-Nexus sourced. | -Nexus sourced. | ||
| Line 248: | Line 260: | ||
Insoluble in FASA (Fumaric Acid Saturated Acetone) | Insoluble in FASA (Fumaric Acid Saturated Acetone) | ||
| + | |||
| + | ==== Bufotenine Benzoate ==== | ||
| + | * Solubility: | ||
| + | Insoluble in acetone, (almost insoluble in PG/VG) | ||
=== Bufotenine Citrate === | === Bufotenine Citrate === | ||
| Line 519: | Line 535: | ||
==== Harmine Citrate ==== | ==== Harmine Citrate ==== | ||
| − | Solubility: | + | *Solubility: |
| − | Soluble in water | + | *Soluble in water |
| − | Insoluble in ethyl acetate | + | *Insoluble in ethyl acetate (will form in EA) |
| + | |||
| + | Nexus sourced | ||
| + | |||
| + | ==== Harmine Tartrate ==== | ||
| + | *Solubility: | ||
| + | |||
| + | *soluble in water | ||
| + | |||
| + | *Insoluble in ethyl acetate (will form in EA) | ||
| + | |||
| + | **Insoluble in acetone (should form in Acetone) | ||
| + | |||
| + | Nexus sourced | ||
=== Harmaline === | === Harmaline === | ||
| Line 569: | Line 598: | ||
Insoluble in ethyl acetate | Insoluble in ethyl acetate | ||
| + | |||
| + | Nexus sourced | ||
| + | |||
| + | ==== Harmaline Tartrate ==== | ||
| + | *Solubility: | ||
| + | |||
| + | *soluble in water | ||
| + | |||
| + | *Insoluble in ethyl acetate (will form in EA) | ||
| + | |||
| + | **Insoluble in acetone (should form in Acetone) | ||
| + | |||
| + | |||
| + | Nexus sourced | ||
=== THH === | === THH === | ||
Latest revision as of 14:18, 19 May 2023
For more detailed information on each compound, please visit their individual wikis as linked in the Alkaloids section
Contents
- 1 Psychedelic Compounds Chemical and Physical Properties
- 2 Relevant NOT psychedelic compounds
- 3 Solvents and Alkaloids XlogP and XlogP3
Psychedelic Compounds Chemical and Physical Properties
DMT
N,N-Dimethyltryptamine
Freebase DMT
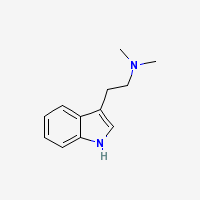 |
- Appearance: White/Transparent crystals or clear oil. Polymorphic (Gaujac et al 2013)
- CAS Registry Number: 61-50-7
- Composition: C12H16N2
- Molecular Weight: 188.26884 g/mol
- Melting point: 45-46 and 57-58°C , Polymorphic (Gaujac et al 2013)
- Boiling point: Source: Brennendes Wasser
First fumes from 100 °C +
Strong fumes from 160 °C +
No further vaporization from 190 °C
Estimated optimal temp for vaporization 175°C
- XLogP: 2.0
- XLogP3: 2.5 (PubChem)
- pKa: 8.68 (Merck Index)
- Colorimetric reagent results: Here
- Stability/Degradation: Oxidation to DMT N-Oxide (yellow oil) in extended presence of oxygen (specialy in evaporation of dmt-containing solvents with heat and/or fan or generally in prolonged exposure to open air). N-oxide may be reverted back to the parent compound by reduction, as described in the N-Oxide to Freebase Wiki.
- Solubility:
Very Soluble in Xylene, Toluene, Limonene, acetone, Isopropyl Alcohol (IPA), methanol, ethanol, Dichloromethane (DCM), chloroform, ether, Butanone (also known as methyl ethyl ketone (MEK)) and butanol.
Soluble in naphtha, hexane, heptane but almost insoluble in these solvents at freezing temperatures. Following daya from Source: Brennendes Wasser
- Naphtha (80 °C, C6-C7, 60-80 °C) "infinite" (the DMT will melt and just create a homogeneous liquid-liquid solution. In the end it may even be a solution of Naphtha in DMT)
- Naphtha (20 °C, C6-C7) 2,93 g in 100 ml
- Naphtha in the freezer (-20 °C, C6-C7) 109 mg in 100 ml
- n-Hexane (20 °C) 770 mg in 100 ml
- n-Hexane in the freezer (-20 °C) 83 mg in 100 ml
- n-Pentane in the freezer (-20 °C) 50 mg in 100 ml
- n-Heptane in the freezer (-20 °C) 92 mg in 100 ml
Almost insoluble in water.
DMT N-Oxide
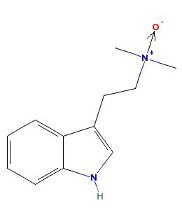 |
- Appearance: Basic oil (Weak base. Weaker than DMT, NMT or tryptamine.) Fish et al. 1956. Hygroscopic solid Banerjee & Ghosal 1969
- XLogP3: 2
- Colorimetric reagent results: Here
- Solubility:
Soluble in Xylene, Toluene, Limonene (?) Soluble in chloroform Bane1 and Ghosal 1969 Soluble in water. Fish et al. 1955 and Ghosal et al. 1970b and Banerjee & Ghosal 1969
Insoluble in petroleum (naphtha) but appreciably soluble in petroleum which contains fats. Ghosal & Banerjee 1969
- Interconversion: It has been claimed that DMT N-Oxide can be reconverted back to DMT by redissolving in dilute acetic acid solution, adding excess zinc dust, mixing for a couple of hours, filtering to remove zinc, adding base and pulling with organic solvent as in a normal extraction. DMT can be oxidized by using hydrogen peroxide (Fish et al 1955)
DMT Fumarate
- Molecular Weight: 492.608 g/mol
- Solubility:
Very soluble in water
Soluble in methanol (~10mg/ml)
Soluble in boiling IPA, Practically insoluble in room temp IPA (~1mg/ml), Insoluble in freeze-cold IPA.
Slightly soluble in ethanol (~5mg/ml)
Insoluble in cold acetone
Insoluble in FASI (Fumaric Acid Saturated IPA)
Insoluble in FASA (Fumaric Acid Saturated Acetone)
DMT Citrate
- Appearance: Oily forms a gum.
- Solubility: Soluble in water, propylene glycol
- Insoluble in ethyl acetate
-Nexus sourced.
DMT Benzoate
- Appearance: Crystal
- Solubility: Soluble in water and ethanol
- Mostly soluble in Isopropyl alcohol (at room temperature)
- Poorly soluble in room temp propylene glycol
- Insoluble: Limonene (but will from in Limonene FB + Benzoic acid)
- Appears to not form in ethyl acetate but is mostly insoluble in ethyl acetate? (at room temperature.)
- Appears to be mostly insoluble in Acetone and MEK at room temperature.
-Nexus sourced.
NMT
N-Methyltryptamine, monomethyltryptamine
Freebase NMT
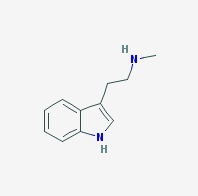 |
- Appearance: Oil, difficult crystallization, eventually forms crystalline stellar aggregates, darkens with exposure to air (Source 1, Source 2)
- Composition: C11H14N2
- Molecular Weight: 174.24226 g/mol
- Melting point: 87-89C (Sigma Aldrich)
- Boiling point: 336.181 °C at 760 mmHg (Chemspider)
- XLogP3: 2.1 (PubChem)
- Colorimetric reagent results: Here
- Stability/Degradation: Darkens over time, but does not seem to form oxides (Source )
- Solubility:
Soluble in methanol, warm ethanol, dichloromethane & choloroform. Soluble to some extent in naphtha (not nearly as much as DMT). It seemed only partially soluble in warm acetic acid. It is likely soluble in xylene. (Source )
- Pharmacology and activity:
-Present in trace amounts as part of normal metabolism (Source)
- 1/3 to 1/4 potency of DMT Nen (2001))
- Further info: TIHKAL NMT entry
5-MeO-DMT
5-methoxy-N,N-dimethyl-tryptamine
Freebase 5-MeO-DMT
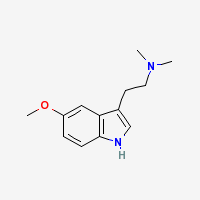 |
- Appearance: Off-white crystals (Sigma Aldrich)
- Composition: C13H18N20
- Molecular Weight: 218.298 g/mol
- Melting Point: 66-67°C, 67-68°C, 69-70°C (Trout's notes and TIHKAL)
- Boiling Point4 : 208-210°C @ 4mm (Hoshino & Shimodaira 1936)
- XLogP: 1.9
- XLogP3: 1.5 (PubChem)
- pKa: 9.3 (Ghosal & Mukherjee 1964)
- Colorimetric reagent results: Here
- Stability: Stable under normal temperatures and pressures. Incompatible with strong oxidizing agents, strong acids (Source)
- Solubility:
Soluble in: Chloroform, ether, DCM, acetone, methanol, ethanol (Trout's notes on simple tryptamines). Soluble in (at least) 20mg/ml 96% ethanol (Source).
Hexane/Naphtha/Pet ether are commonly used to crystallize it (Ott 1993, Espamer et al 1967, Morimoto & Matsumoto 1966, etc) , so it is probably moderately soluble in these solvents.
Practically Insoluble in water (Source)
5-MeO-DMT Hydrochloride
- Melting Point: 145-146°C (TIHKAL)
Bufotenine
5-HO-DMT - 5-hydroxy-N,N-dimethyl-tryptamine
Freebase Bufotenine
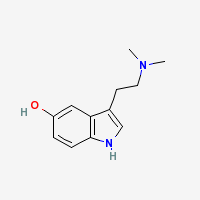 |
- CAS Registry Number: 487-93-4
- Composition: C12H16N2O
- Molecular Weight: 204.268 g/mol
- Melting point:Source: Brennendes Wasser
108 °C
- Boiling point:Source: Brennendes Wasser
First fumes from 160 °C +
Bufotenine turning brown at 170 °C +
strong fumes from 190 °C +
No further vaporization from 230 °C, leaving a black residue
Estimated optimal temp for vaporization 210°C
1:3 Ethyl Acetate:Naphtha (C6-C7)
Boiling = 7,6 g/l = 760 mg in 100 ml
20 °C = 3,6 g/1 l = 360 mg in 100 ml
- 20 °C = 3,5 g/1 l = 350 mg in 100 ml
Selectively dissolves Bufotenine only, not the more polar Alkaloids. Defat first with Naphtha.
Ethyl Acetate
Boiling = 280 g/1 l = 28 g in 100 ml
20 °C = 72 g/1 l = 7,2 g in 100 ml
- 20 °C = 50 g/1 l = 5 g in 100 ml
For recrystalization (optional, not recommended). Use only minimal amounts of solvent as you can see above! Placement in the freezer did not produce more crystals, just cloudings that cant be collected. Also crystals derived from re-x with Ethyl Acetate start turning grey and otherwise the material keeps its colour, therefore maybe not advised to do a re-x.
boiling D-Limonene
Unconvenient, drops Bufotenine too fast to form crystals. Also high chance to damage Bufotenine at 175 °C. Same goes possibly for Xylene.
1:4 Acetone:Naphtha
Not recommended, check above link for more info
Other solubility data (possibly unreliable, originally from 69ron experiments)
Acetone @ 20 C: soluble (5 g/100 ml)
Chloroform @ 20 C: soluble
Dichloromethane @ 20 C: soluble
Dimethyl sulfoxide (DMSO) @ 20 C: soluble (6 g/100 ml)
D-Limonene (Orange Oil) @ 20 C: insoluble
D-Limonene (Orange Oil) @ 176 C: soluble (more than 1.7 g/100 ml)
Dilute Acids and Alkalis: Soluble (Merck Index)
Ethanol @ 20 C: soluble
Ether @ 20 C: soluble
Ethyl acetate @ 20 C: soluble
Heptane @ 20 C: insoluble
Heptane with 40% MEK @ 20 C: soluble (0.53 g/100 ml)
Heptane with 50% MEK @ 20 C: soluble (1.22 g/100 ml)
IPA @ 20 C: soluble
MEK @ 20 C: soluble
Methanol @ 20 C: soluble
Naphtha @ 20 C: insoluble
Water @ 20 C: nearly insoluble in pure water (no acid or alkali added)
Xylene @ 20 C: nearly insoluble (less than 0.03 g/100 ml)
Xylene @ 144 C: soluble (1.5 g/100 ml)
Bufotenine Fumarate
- Solubility:
Soluble in water
Insoluble in FASA (Fumaric Acid Saturated Acetone)
Bufotenine Benzoate
- Solubility:
Insoluble in acetone, (almost insoluble in PG/VG)
Bufotenine Citrate
- Insoluble in ethyl acetate (will form in EA)-Nexus sourced.
Psilocin
4-HO-DMT - 4-Hydroxy-N,N-dimethyl-tryptamine
Freebase Psilocin
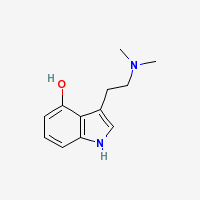 |
- CAS Registry Number: 520-53-6
- Composition: C12H16N2O
- Molecular Weight: 204.27 g/mol
- Melting Point: 103-104°C (TIHKAL) , 173-176°C (Merck Index)
- XLogP3: 2.1 (PubChem)
- Colorimetric reagent results: Here
- Stability/Degradation: Unstable in solution, especially alkaline solution (Merck Index).
- Solubility:
Soluble in 70% ethanol. Poorly soluble in dry ethanol, and poorly soluble in ethanol less than 60%. Very slightly soluble in water (sources: Merck Index , scientific publications)
Psilocybin
4-PO-DMT - O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine
Psilocybin (Psilocin Phosphate Ester)
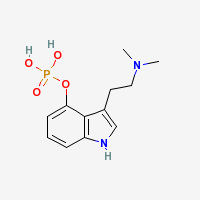 |
- CAS Registry Number: 520-52-5
- Composition: C12H17N2O4P
- Molecular Weight: 284.248141 g/mol
- Melting Point: 220-228° from boiling water; 185-195° from boiling methanol (Merck Index)
Free base: Stable compound. Colorless crystals. Hofmann 1971 pH 5.2 (in 50% EtOH) Merck 9th More stable than psilocin. Shulgin & Shulgin !997 mp 175-180° Repke & leslie 1977 mp 185-195° (from boiling methanol) Ott 1996 mp 185-195° (from methanol) Picker & Rickards 1970 (Also in Perkal 1981) rnp 185-195° (dec.) (white crystals) Clarke 's 1986 mp 190° Mantle & Waight 1969
- XLogP3: -1.6 (PubChem)
- Colorimetric reagent results: Here
- Stability/Degradation: Dephosphorylated into Psilocin under acidic conditions. Also when ingested, by phosphatases enzymes.
- Solubility:
Soluble in water (estimated 2mg/ml at 25C). Soluble in 20 parts boiling water (= 0.79g/ml in boiling water), 120 parts boiling methanol (= 0.0932g/ml in boiling methanol); very soluble in 70% methanol saturated with KNO3, soluble in dry methanol, difficultly soluble in ethanol, increasingly less soluble in methanol less than 80%. Practically insoluble in chloroform, benzene (sources: Merck Index , scientific publications, PubChem)
Mescaline
3,4,5-trimethoxyphenethylamine
Freebase Mescaline
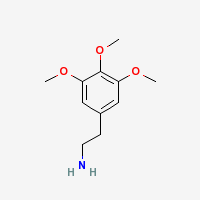 |
- CAS Registry Number: 54-04-6
- Composition: C11H17NO3
- Melting point: 35-36°C (Kindler and Peschke, 103. Merck Index)
- Boiling point: 180°C (12 mmHg)
- XLogP: 0.6 (PubChem)
- XLogP3: 0.7 (PubChem)
- pKa: 9.56
- Appearance: long needle shaped white crystals
- Molecular weight: 211.26
Notes: forms mescaline carbonate on prolonged exposure to air
- Average dose: 300 to 600 milligrams with a duration of 5 to 12 hours.
- Colorimetric reagent results: Here
- Solubility
Soluble in: alcohol, chloroform, benzene, xylene, toluene, acetone, dichloromethane, highly soluble in isopropyl alcohol, soluble in d-limonene, ethyl acetate, chilled ethyl acetate (O°F)
Moderately soluble in: water
Insoluble in: practically insoluble in ether or petroleum ether
- LD50: i.p. rats 370 mg/kg
Mescaline Citrate
- Solubility
Soluble in water
Insoluble in: xylene, acetone *, ethyl acetate
* Possible unreliable web source.
Mescaline Hydrochloride
 |
- Molecular weight: 247.72 (Sigma Aldrich)
- Empirical Formula (Hill Notation): C11H17NO3 • HCl (Sigma Aldrich)
- CAS Number: 832-92-8
- Appearance: colorless crystals, needles
- Melting point: 184°C (Merck Index)
- Solubility:
Moderately soluble in: water, ethanol ~10mg/ml (ref: Cayman Chemical) (Merck Index), methanol ~1.0mg/ml (source Sigma Aldrich solution) (Merck Index), dimethyl sulfoxide (DMSO) ~3mg/ml (ref: Cayman Chemical), dimethyl formamide ~0.5mg/ml (ref: Cayman Chemical)
Insoluble in: practically insoluble in toluene and acetone, insoluble in isopropyl alcohol, diethyl ether, and d-limonene
- LD50: i.p. rats 132 mg/kg
- Storage temperature: 2-8°C (Sigma Aldrich)
- Isolation: when mescaline hydrochloride is extracted from San Pedro, Achuma, or Peruvian torch, it can be isolated from the other alkaloids by washing it in IPA or acetone (use 10 ml per gram of alkaloids, and wash 2-3 times). The non-mescaline alkaloids dissolve in the IPA or acetone (* NOTE: previous sentence is speculation, not yet substantiated by tests as far as we know), while the mescaline hydrochloride does not. Note that for the cleanest results use about 2 washes of acetone, and then 2 washes with IPA.
Mescaline Picrate
- Melting point: mp 222°C.
Mescaline Sulfate Dihydrate
 |
- Composition: (C11H17NO3)2 • H2SO4 • 2H2O
- Appearance: prisms
- Melting point: 183–186 °C (361–367 °F)
- Molecular Weight: 309.33606
- Soluble in: hot water, methanol
- Almost insoluble in: near freezing water, alcohol, acetone * [VERIFIED]
* Possible unreliable web source.
Mescaline Fumarate
- Composition: unknown (when prepared by a FASx method it may be a one to one salt (the bifumarate) or may not be)
- Appearance: White powder
- Melting point: unknown
- Solubility
Soluble in: water
Insoluble in: Limonene, Anydrous IPA, Acetone, MEK (most likely insoluble in all non-polars like xylene and toluene) Source
Mescaline Acetate
- Composition: unknown
- Appearance: white free flowing powder with a slight waxy texture ***
- Melting point: unknown
- Solubility:
Soluble in: water***, isopropyl alcohol***, acetone***, DMSO*** (more than 5 grams/100 ml), boiling MEK***
Insoluble in: xylene, d-limonene, cold MEK (Methyl Ethyl Ketone) ***
*** This information was validated by SWIM and is reliable.
- Isolation: when mescaline acetate is extracted from San Pedro, Achuma, or Peruvian torch, it can be isolated from the other alkaloids by washing it in cold MEK (use 10 ml per gram of alkaloids, and wash 2-3 times). The non-mescaline alkaloids dissolve in the MEK, while the mescaline acetate does not. Mescaline acetate can be recrystallized in MEK by boiling the MEK and then freezing it overnight.
Ibogaine
12-methoxyibogamine
Freebase Ibogaine
 |
- CAS Registry Number: 83-74-9
- Composition: C20H26N2O
- Molecular Weight: 310.43324 g/mol
- Melting Point: 152-153 °C (TIHKAL)
- XLogP3: 3.9 (PubChem)
- pKa: 8.1 in 80% methylcellosolve (Merck Index)
- Colorimetric reagent results: Here
- Solubility: Soluble in limonene (Non-Toxic Iboga Extraction) soluble in acetone (merck)
Ibogaine Hydrochloride
- Melting Point: 299-300 °C (TIHKAL)
- Solubility: Soluble in water, methanol (Sigma Aldrich), slightly soluble in acetone (merck)
Voacangine
12-methoxyibogamine-18-carboxylic acid methyl ester
Voacangine
 |
- Composition: C22H28N2O3
- Molecular Weight: 368.46932 g/mole
- Melting Point: 136–137 °C (Merck Index)
- pKa: 7.4 (40% aq methanol); 5.73 (33% DMF) (Merck Index)
- XLogP3-AA: 3.5 (PubChem)
LSA (ergine)
Lysergamide
Freebase LSA
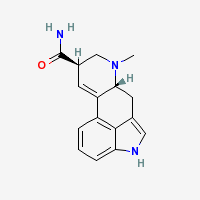 |
CAS Registry Number: 478-94-4
- Composition: C16H17N3O
- Molecular Weight: 267.32568 g/mol
- XLogP3: 1.6 (PubChem)
Muscimol
Pantherine, Agarine
Freebase Muscimol
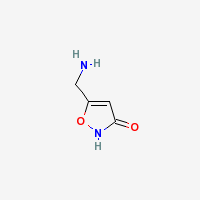 |
- Appearance: White powder (Sigma Aldrich)
- CAS Registry Number: 2763-96-4
- Composition: C4H6N2O2
- Molecular Weight: 114.10264 g/mol
- Melting Point: 175°C (Merck Index)
- Boiling Point: 70°C (Wolfram Alpha)
- XLogP3-AA: -1.4 (PubChem)
- pKa: 4.8 (Indiana University])
- Solubility: Very soluble in water and methanol, slightly soluble in ethanol, DMSO and DMF. ~10mg/ml in phosphate buffered saline @ pH 7.2 (Caymanchem.com)
Salvinorin A
Note: Technically NOT an alkaloid, as it contains no nitrogen. It is a trans-neoclerodane diterpene.
Salvinorin A
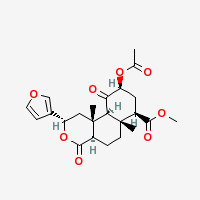 |
- Composition: C23H28O8
- Molecular Weight: 432.46362 g/mol
- Melting Point:
242-244 – 238-240 °C (Wikipedia)
no melting, directly sublimating source: Brennendes Wasser
- Boiling Pont:
First fumes from 190 °C, Salvinorin turning brown
No further vaporization from 270 °C, leaving a black residue
Estimated ideal temperature for vaporization: 250 °C
Other data: 760.2 °C (1400 °F) (Wikipedia) likely wrong
- XLogP3-AA: 2.5 (PubChem)
Elemicin
1,2,3-trimethoxy-5-allylbenzene
Note: Technically NOT an alkaloid, as it contains no nitrogen.
Elemicin
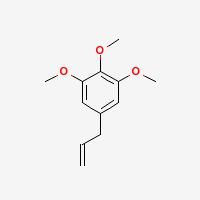 |
- CAS Number: 487-11-6
- Molecular Formula: C12H16O3
- Molecular Weight: 208.25364 g/mol
- Boiling Point: 152-156 °C @ 17 mmHg, 144-147 °C @ 10 mmHg (Source), 146-147 °C (Source 1 Source 2), 279.8 °C @ 760 mmHg (ChemSpider)
- XlogP3: 2.5 (PubChem)
- Solubility:
Soluble in alcohol, Insoluble in water (Source)
Harmine
7-methoxy-β-carboline
Freebase Harmine
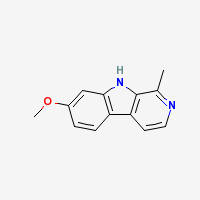 |
- Composition: C13H12N2O
- Molecular Weight: 212.25 (Sigma Aldrich)
- Melting point:
262-264 °C (Sigma Aldrich)
Not melting, directly sublimating Source: Brennendes Wasser
- Boiling point:
First fumes from 180 °C +
Sublimation while forming small black clumps at 205 °C +
No further vaporization from 240 °C,
421.4°C at 760mmHg (Lookchem)
- XLogP: 2.5
- XLogP3: 3.6
- pKa: 7.7
- Colorimetric reagent results: Here
- Solubility: ethanol (~1.5mg/ml), DMSO (~1.5mg/ml), dimethyl formamide and ethyl acetate
(~1.5mg/ml). Reasonably soluble in acetone (at 25°C, acetone can dissolve 4mg/ml mixed harmalas as this test shows) Insoluble in: Basic water, diethyl ether, D Limonene. Low solubility in distilled water.
- Isolation: To separate from harmaline, using pKa properties, raise pH of solution containing both alkaloids to pH 8.75 to precipitate 92% of harmine and only 8% Harmaline. Filter to retrieve precipitated alkaloids, and raise the pH further to retrieve the bulk of harmaline. Check the freebase percentage calculator thread and the Harmala Extraction Guide for more info.
Harmine Hydrochloride
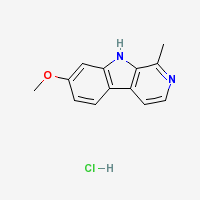 |
- Melting Point: 321 °C
- Solubility:
Soluble in water
Insoluble in salt-saturated water
Harmine Citrate
- Solubility:
- Soluble in water
- Insoluble in ethyl acetate (will form in EA)
Nexus sourced
Harmine Tartrate
- Solubility:
- soluble in water
- Insoluble in ethyl acetate (will form in EA)
- Insoluble in acetone (should form in Acetone)
Nexus sourced
Harmaline
3,4-dihydro-7-methoxy-1-methyl-β-carboline
Freebase Harmaline
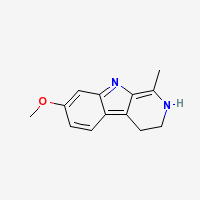 |
- Composition: C13H14N2O
- Melting point:
232-234 °C (Sigma Aldrich)
Not melting, directly sublimating Source: Brennendes Wasser
- Boiling point:
First fumes from 180 °C +
Sublimation while forming small black clumps at 205 °C +
No further vaporization from 240 °C,
120-140 °C at 0.001 mm/Hg (TIHKAL)
- XLogP: 0.8
- XLogP3: 1.2
- pKa: 9.8
- Colorimetric reagent results: Here
- Solubility:
Insoluble in D Limonene Slightly soluble in basic water, poorly soluble in distilled water. Reasonably soluble in acetone (at 25°C, acetone can dissolve 4mg/ml mixed harmalas as this test shows) Soluble in ethyl acetate.
- Isolation: To separate from harmine, using pKa properties, raise pH of solution containing both alkaloids to pH 8.75 to precipitate 92% of harmine and only 8% Harmaline. Filter to retrieve precipitated alkaloids, and raise the pH further to retrieve the bulk of harmaline. Check the freebase percentage calculator thread and the Harmala Extraction Guide for more info.
Harmaline Hydrochloride
- Solubility:
Soluble in water
Insoluble in salt-saturated water
Harmaline Citrate
Solubility:
Soluble in water
Insoluble in ethyl acetate
Nexus sourced
Harmaline Tartrate
- Solubility:
- soluble in water
- Insoluble in ethyl acetate (will form in EA)
- Insoluble in acetone (should form in Acetone)
Nexus sourced
THH
Tetrahydroharmine - 7-methoxy-1-methyl-1,2,3,4-tetrahydro-β-carboline
Freebase THH
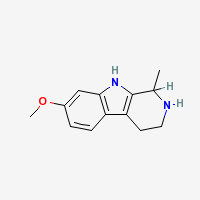 |
- Composition: C13H16N2O
- Molecular Weight: 216.27894 g/mol
- Melting Point: 187-190 °C (TIHKAL)
- XlogP: 1.9
- XLogP3: 1.9
- Colorimetric reagent results: Here
- Solubility:
Soluble in chloroform, ethanol & methanol. (Ott 1996.)
Soluble in ethyl acetate. (Siddiqui et al. 1983)
Poorly soluble in distilled water
THH Hydrochloride
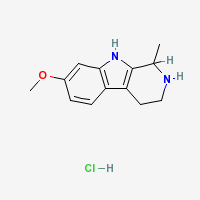 |
- Composition: C13H17ClN2O
- Molecular Weight: 252.73988 g/mol
- Melting Point: 232–234 °C (TIHKAL)
- Boiling point: 399.2 °C at 760 mmHg (Lookchem)
- Mol File: 40959-16-8.mol
- Flash Point: 195.2 °C
- Enthalpy of Vaporization: 64.99 kJ/mol
- Vapour Pressure: 1.4E-06 mmHg at 25°C
- H-Bond Donor: 3
- H-Bond Acceptor: 2
- Rotatable Bond Count: 1
- Topological Polar Surface Area: 37.1
- Heavy Atom Count: 17
- Complexity: 258
Relevant NOT psychedelic compounds
Gramine
N,N-Dimethyl-1H-indole-3-methanamine
Freebase Gramine
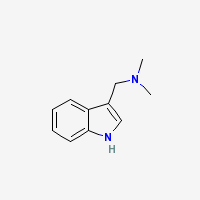 |
- Composition: C11H14N2
- Molecular Weight: 174.24226 g/mol
- Melting point: 138-139°C (Merck)
- Boiling point:
- XLogP3: 1.8
- Colorimetric reagent results: Here
- pKa: 8.52
- Solubility:
Sol in alcohol, ether, chloroform; slightly sol in cold acetone. Practically insol in petr ether, water. (Merck Index)
Hordenine
N,N-dimethyltyramine
Freebase Hordenine
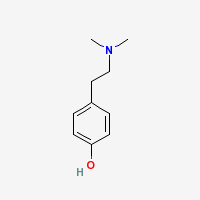 |
- CAS Registry Number: 539-15-1
- Composition: C10H15NO
- Molecular Weight: 165.23 g/mol
- Melting point: 117-118° (Merck Index)
- Boiling point: 173° @ 11mmHg (Merck Index)
- XlogP3: 2.1 (PubChe[[File:m)
- Solubility:
Very sol in alcohol, chloroform, ether. 7 grams dissolve in 1000 ml water. Sparingly sol in benzene, toluene, xylene. Practically insol in petr ether. (Merck Index)
Hordenine Hydrochloride
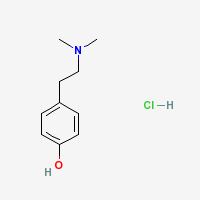 |
- CAS Registry Number: 6027-23-2
- Composition: C10H15NO.HCl
- Molecular Weight: 201.70 g/mol
- Melting point: 177° (Merck Index)
- Solubility:
Very sol in water. (Merck Index)
Vasicine
(3R)-1,2,3,9-tetrahydropyrrolo[2,1-b]quinazolin-3-ol;Peganine
Freebase Vasicine
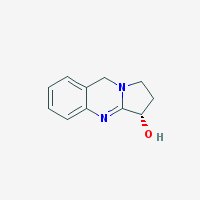 |
- Composition: C11H12N2O
- Molecular Weight: 188.22578 g/mol
- Melting point: 209-211° C (Decomposes) (source)
- XLogP3: 0.4 (pubchem)
- Pharmacology: Uterotonic, abortifacient, bronchodilatory activity, expectorant, respiratory stimulant activity, moderate hypotensive activity (sources: 1, 2
- Solubility: Soluble in NaCl-saturated water (harmalas precipitate as HCl salts in NaCl-saturated water, hence vasicine can be separated from harmalas in peganum harmala. Check harmala extraction guide for more info)
Vasicinone
(R)-2,3-dihydro-3-hydroxypyrrolo(2,1-b)quinazolin-9(1H)-one
Freebase Vasicinone
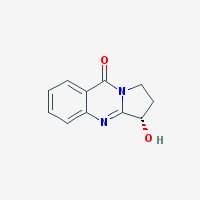 |
- Appearance: White powder
- Composition: C11H10N2O2
- Molecular Weight: 202.2093 g/mol
- Melting point: 203°-204° C (source)
- XLogP3: 0.4 (pubchem)
- Pharmacology: Uterotonic, abortifacient, bronchodilatory activity, expectorant, respiratory stimulant activity, moderate hypotensive activity (sources: 1, 2
- Solubility: Water: 1600 mg/l. Soluble in NaCl-saturated water (harmalas precipitate as HCl salts in NaCl-saturated water, hence vasicinone can be separated from harmalas in peganum harmala. Check harmala extraction guide for more info
Deoxyvasicine
1,2,3,9-Tetrahydropyrrolo(2,1-b)quinazoline; Deoxypeganin;
Freebase Deoxyvasicine
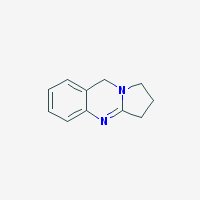 |
- Appearance: White to yellow powder
- Composition: C11H12N2
- Molecular Weight: 172.22638 g/mol
- Melting point: 86-87° C (source)
- XLogP3: 0.4
- Pharmacology: Uterotonic, abortifacient, bronchodilatory activity, expectorant, respiratory stimulant activity, moderate hypotensive activity (sources: 1, 2
- Solubility: Water: 1600 mg/l. Soluble in NaCl-saturated water (harmalas precipitate as HCl salts in NaCl-saturated water, hence vasicine can be separated from harmalas in peganum harmala. Check harmala extraction guide for more info)
Solvents and Alkaloids XlogP and XlogP3
Alkaloid XlogP list
- 0.1 - 6-methoxy-2-methyl-beta-Carboline
- 0.7 - Beta-carboline, 6-methoxy-1,2-dimethyl-1,2-Dimethyl-2H-beta-carbolin-6-yl methyl ether
- 0.7 - Mescaline
- 0.8 - Harmaline
- 1.0 - 5-HO-Tryptamine (serotonin)
- 1.3 - 5-HO-DMT N-oxide (Bufotenine N-oxide)
- 1.6 - 5-HO-DMT (bufotenine)
- 1.7 - N-Methylserotonin
- 1.7 - DMT N-oxide (Dimethyltryptamine N-oxide)
- 1.7 - 5-MeO-NMT (5-Methoxy-N-methyltryptamine)
- 1.7 - 2-Methyl-1,2,3,4-tetrahydro-beta-carboline
- 1.8 - NMT (N-Methyltryptamine)
- 1.9 - 5-MeO-DMT (methoxybufotenin)
- 1.9 - Tetra−Hydro−Harmine (THH)
- 2.0 - DMT (Dimethyltryptamine)
- 2.5 - Harmine
Alkaloid XlogP3 list
- -1.4 - Muscimol
- -1.6 - Psilocybin
- 0.6 - Harmalol
- 0.7 - Mescaline
- 1.2 - Harmaline
- 1.2 - Bufotenine
- 1.5 - 5-MeO-DMT
- 1.6 - LSA (ergine)
- 1.8 - Gramine
- 1.9 - Tetrahydroharmine
- 2.0 - DMT N-oxide
- 2.1 - Psilocin
- 2.1 - NMT
- 2.5 - DMT
- 2.5 - Salvinorin A
- 2.5 - Elemicin
- 3.5 - Voacangine
- 3.6 - Harmine
- 3.6 - Harman
- 3.9 - Ibogaine
Lower XlogP values are more water soluble, and higher XlogP values are more non-polar soluble.
Solvent XlogP list
- -0.7 - DMSO
- -0.5 - Methanol
- -0.1 - Ethyl Alcohol
- 0.2 - Acetone
- 0.4 - IPA
- 0.4 - MEK (Methyl Ethyl Ketone)
- 0.7 - Ethyl Acetate
- 0.9 - Ethyl Ether
- 1.5 - DCM
- 2.1 - Chloroform
- 2.5 - Toluene
- 2.5 - Xylene
- 3.7 - Limonene
- 4.3 - Heptane (similar to naphtha)