Difference between revisions of "Solvent physical properties"
Mindlusion (Talk | contribs) (→Solvents) |
Mindlusion (Talk | contribs) (→Dichloromethane (DCM)) |
||
| (24 intermediate revisions by one user not shown) | |||
| Line 23: | Line 23: | ||
===Water=== | ===Water=== | ||
| + | |||
| + | [[File:Water.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |Water || 100 °C || Miscible || 1.000 g/ml || 18.02 g/mol || | ||
| + | |- | ||
| + | |} | ||
===Carboxylic acids=== | ===Carboxylic acids=== | ||
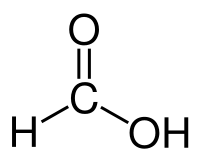
====Formic acid==== | ====Formic acid==== | ||
| + | |||
| + | [[File:Formicacid.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |Formic acid || 100.8 °C || Miscible || 1.22 g/ml || 46.03 g/mol || | ||
| + | |- | ||
| + | |} | ||
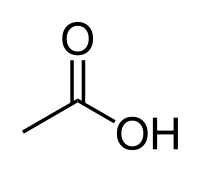
====Acetic acid==== | ====Acetic acid==== | ||
| + | [[File:Aceticacid.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |Acetic acid || 118 °C || Miscible || 1.049 g/ml || 60.05 g/mol || | ||
| + | |- | ||
| + | |} | ||
===Alcohols=== | ===Alcohols=== | ||
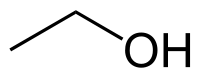
====Ethanol==== | ====Ethanol==== | ||
| + | |||
| + | |||
| + | [[File:Ethanol.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C2H5OH || 79 °C || Miscible || 0.789 g/ml || 46.07 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Azeotrope % weight /w water = 95.5% | ||
| + | |||
| + | Miscible in: | ||
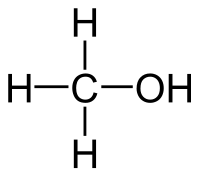
====Methanol==== | ====Methanol==== | ||
| + | |||
| + | [[File:Methanol.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C2H5OH || 65 °C || Miscible || 0.791 g/ml || 32.04 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | No azeotrope w/ water | ||
| + | |||
| + | Miscible in: | ||
====Propanol==== | ====Propanol==== | ||
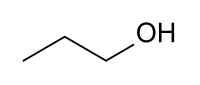
| + | |||
| + | [[File:Propanol.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C2H5OH || 97 °C || Miscible || 0.803 g/ml || 60.1 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Azeotrope % weight /w water = 71.7% | ||
| + | |||
| + | Miscible in: | ||
====Isopropanol==== | ====Isopropanol==== | ||
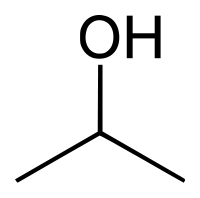
| + | |||
| + | [[File:200px-2-Propanol.svg.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C3H7OH || 82.5 °C || Miscible || 0.786 g/ml || 60.1 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Azeotrope % weight /w water = 87.9% | ||
| + | |||
| + | Miscible in: non-polar solvents, haloforms, water | ||
====Glycerol==== | ====Glycerol==== | ||
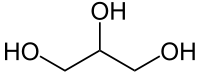
| + | |||
| + | [[File:Glycerol.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C3H5(OH)3 || 290 °C || Miscible || 1.261 g/ml || 92.09 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Azeotrope % weight /w water = | ||
| + | |||
| + | Miscible in: water, alcohols | ||
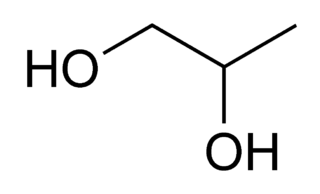
====Propylene Glycol==== | ====Propylene Glycol==== | ||
| + | [[File:propyleneglycol.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C3H7OH || 188.2 °C || Miscible || 1.036 g/ml || 76.09 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Azeotrope % weight /w water = | ||
| + | |||
| + | Miscible in: | ||
==Polar aprotic== | ==Polar aprotic== | ||
===Ketones=== | ===Ketones=== | ||
| + | |||
| + | Short chain ketones are miscible in ALL organic solvents | ||
| + | |||
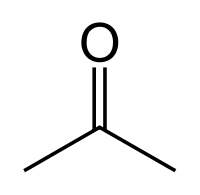
====Acetone==== | ====Acetone==== | ||
| + | |||
| + | [[File:Acetone.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Formula | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C3H6O || 56 °C || Miscible || 0.786 g/ml || 58.08 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Miscible in: | ||
| + | |||
| + | All organic solvents | ||
| + | |||
| + | - | ||
| + | Does not from an Azeotrope with water | ||
====Methyl ethyl ketone (MEK)==== | ====Methyl ethyl ketone (MEK)==== | ||
| + | |||
| + | [[File:MEK.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Formula | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C4H8O || 80 °C || Miscible 275. g/L || 0.805 g/ml || 72.11 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Miscible in: | ||
| + | |||
| + | All organic solvents | ||
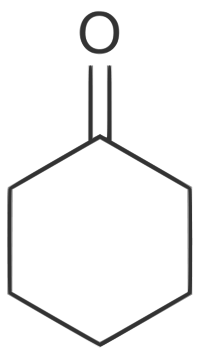
====Cyclohexanone==== | ====Cyclohexanone==== | ||
| + | |||
| + | [[File:Cyclohexanone.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Formula | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C6H10O || 155.65 °C || Miscible 90 g/L || 0.9478 g/ml || 98.15 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Miscible in: | ||
| + | |||
| + | All organic solvents | ||
===Esters=== | ===Esters=== | ||
| Line 109: | Line 295: | ||
====Chloroform==== | ====Chloroform==== | ||
| + | |||
| + | [[File:Chloroform.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C2H5OH || 61.2 °C || Immiscible || 1.483 g/ml || 199.38 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Azeotrope % weight /w water = 97.0% | ||
| + | |||
| + | Miscible in: non-polar solvents, alcohols | ||
====Carbon Tetrachloride==== | ====Carbon Tetrachloride==== | ||
====Dichloromethane (DCM)==== | ====Dichloromethane (DCM)==== | ||
| + | |||
| + | [[File:DCM.png]] | ||
| + | {| class="wikitable" style="text-align: center; color: green;" | ||
| + | !Solvent | ||
| + | !Boiling Point °C | ||
| + | !Solubility /w Water | ||
| + | !Density | ||
| + | !Molar mass | ||
| + | |- | ||
| + | |C2H5OH || 39.6 °C || Immiscible || 1.33 g/ml || 84.93 g/mol || | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | Azeotrope % weight /w water = 98.5% | ||
| + | |||
| + | Azeotrope % weight /w methanol = 7.3% | ||
| + | |||
| + | Azeotrope % weight /w diethyl ether = 30% | ||
| + | |||
| + | Miscible in: non-polar solvents, alcohols | ||
===Other=== | ===Other=== | ||
| Line 119: | Line 341: | ||
| + | |||
| + | |||
| + | =Common Azeotropes= | ||
| + | |||
| + | (taken from wikipedia) | ||
{| | {| | ||
| − | | | + | | align="center" valign="top" rowspan="2" | <big>'''Azeotropes of water, b.p.=100 °C'''</big> |
| + | {| class="wikitable" | ||
| + | ! 2nd Component !! b.p. of<br>comp. (˚C) !! b.p. of<br>mixture (˚C) !! % by<br>weight !! spef.<br>grav | ||
|- | |- | ||
| − | | | + | | colspan="5" align="center" | '''with various [[alcohol]]s''' |
|- | |- | ||
| − | | | + | | [[ethanol]] || 78.4 || 78.1 || 95.5 |
| + | | bgcolor="#FFFFCC" | 0.804 | ||
| + | |- bgcolor="#CCFFFF" | ||
| + | | [[methanol]]<ref name="br"/> || 64.7 | ||
| + | | colspan="3" align="center" | No azeotrope | ||
| + | |- | ||
| + | | [[Propan-1-ol|''n''-propanol]] || 97.2 || 87.7 || 71.7 | ||
| + | | bgcolor="#FFFFCC" | 0.866 | ||
| + | |- | ||
| + | | [[Propan-2-ol|''iso''-propanol]] || 82.5 || 80.4 || 87.9 | ||
| + | | bgcolor="#FFFFCC" | 0.818 | ||
| + | |- | ||
| + | | [[n-Butanol|''n''-butanol]] || 117.8 || 92.4 || 55.5<br>U 79.9<br>L 7.7 | ||
| + | | bgcolor="#FFFFCC" | <br>U 0.849<br>L 0.990 | ||
| + | |- | ||
| + | | [[2-Butanol|''sec''-butanol]] || 99.5 || 88.5 || 67.9 | ||
| + | | bgcolor="#FFFFCC" | 0.863 | ||
| + | |- | ||
| + | | [[isobutanol|''iso''-butanol]] || 108.0 || 90.0 || 70.0<br>U 85.0<br>L 8.7 | ||
| + | | bgcolor="#FFFFCC" | <br>U 0.839<br>L 0.988 | ||
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[Tert-Butanol|''tert''-butanol]] || 82.8 || 79.9 || 88.3 || | ||
| + | |- | ||
| + | | [[allyl alcohol]] || 97.0 || 88.2 || 72.9 | ||
| + | | bgcolor="#FFFFCC" | 0.905 | ||
| + | |- | ||
| + | | [[benzyl alcohol]] || 205.2 || 99.9 || 9 || | ||
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[furfuryl alcohol]] || 169.4 || 98.5 || 20 || | ||
| + | |- bgcolor="#CCFFFF" | ||
| + | | [[cyclohexanol]]<ref name="metalfinishing"/> || 161.1 || 97.8 || 20 || | ||
| + | |- bgcolor="#CCFFFF" | ||
| + | | [[benzyl alcohol]]<ref name="metalfinishing"/> || 205.4 || 99.9 || 9 || | ||
| + | |- | ||
| + | | colspan="5" align="center" | '''with various [[organic acid]]s''' | ||
| + | |- | ||
| + | | [[formic acid]] || 100.8 || 107.3 || 77.5 || | ||
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[acetic acid]] <sup>‡</sup><ref name="br">{{Cite web | ||
| + | | url = http://www.solvent--recycling.com/azeotrope_1.html | ||
| + | | title = What is an Azeotrope? | ||
| + | | accessdate = 24 March 2007 | ||
| + | | author = | ||
| + | | last = | ||
| + | | first = | ||
| + | | authorlink = | ||
| + | | coauthors = | ||
| + | | date = | ||
| + | | year = | ||
| + | | month = | ||
| + | | work = | ||
| + | | publisher = B/R Corporation | ||
| + | | pages = | ||
| + | | language = | ||
| + | | quote = | ||
| + | | archiveurl= http://web.archive.org/web/20070424141816/http://www.solvent--recycling.com/azeotrope_1.html| archivedate= 24 April 2007 <!--DASHBot-->| deadurl= no}}</ref><ref name="hilmen"> | ||
| + | {{Cite web | ||
| + | | url = http://www.chemeng.ntnu.no/thesis/download/2000/hilmen/Thesis_Hilmen.pdf | ||
| + | | title = Separation of Azeotropic Mixtures: Tools for Analysis and Studies on Batch Distillation Operation | ||
| + | | accessdate = 24 March 2007 | ||
| − | + | | author = Eva-Katrine Hilmen | |
| − | | | + | | last = Hilmen |
| + | | first = Eva-Katrine | ||
| + | | authorlink = | ||
| + | | coauthors = | ||
| + | | date = | ||
| + | | year = 2000 | ||
| + | | month = November | ||
| + | | format = | ||
| + | | work = | ||
| + | | publisher = Norwegian University of Science and Technology, dept. of Chemical Engineering | ||
| + | | pages = | ||
| + | | language = | ||
| + | | archiveurl = | ||
| + | | archivedate = | ||
| + | | quote = | ||
| + | }}</ref> || 118.1 | ||
| + | | colspan="3" align="center" | No azeotrope | ||
| + | |- | ||
| + | | [[propionic acid]] || 141.1 || 99.98 || 17.7 | ||
| + | | bgcolor="#FFFFCC" | 1.016 | ||
| + | |- | ||
| + | | [[butyric acid]] || 163.5 || 99.94 || 18.4 | ||
| + | | bgcolor="#FFFFCC" | 1.007 | ||
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[isobutyric acid|''iso''-butyric acid]] || 154.5 || 99.3 || 21 || | ||
| + | |- | ||
| + | | colspan="5" align="center" | '''with [[mineral acid]]s''' | ||
| + | |- | ||
| + | | [[nitric acid]] || 86.0 || 120.5 || 68 | ||
| + | | bgcolor="#FFFFCC" | 1.405 | ||
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[perchloric acid]] || 110.0 || 203 || 71.6 || | ||
| + | |- | ||
| + | | [[hydrofluoric acid]] || 19.9 || 120 || 37 || | ||
| + | |- | ||
| + | | [[hydrochloric acid]] || –84 || 110 || 20.24 | ||
| + | | bgcolor="#FFFFCC" | 1.102 | ||
| + | |- | ||
| + | | [[hydrobromic acid]] || –73 || 126 || 47.5 | ||
| + | | bgcolor="#FFFFCC" | 1.481 | ||
| + | |- | ||
| + | | [[hydroiodic acid]] || –34 || 127 || 57 | ||
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[sulfuric acid]] || 290 || 338 || 98 || | ||
|- | |- | ||
| − | | | + | | colspan="5" align="center" | '''with various [[alkyl halide]]s''' |
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[1,2-Dichloroethane|ethylene chloride]] || 83.7 || 72 || 91.8 || | ||
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[1,2-Dichloropropane|propylene chloride]] || 96.8 || 78 || 89.4 || | ||
| + | |- bgcolor="#FFFFCC" | ||
| + | | [[chloroform]] || 61.2 || 53.3 || 97.0<br>U 0.8<br>L 99.8 | ||
| + | | bgcolor="#FFFFCC" | <br>U 1.004<br>L 1.491 | ||
| + | |- bgcolor="#FFFFCC" | ||
| + | | [[carbon tetrachloride]] || 76.8 || 66.8 || 95.9<br>U 0.03<br>L 99.97 | ||
| + | | bgcolor="#FFFFCC" | <br>U 1.000<br>L 1.597 | ||
| + | |- bgcolor="#FFFFCC" | ||
| + | | [[dichloromethane|methylene chloride]] || 40.0 || 38.8 || 99.6<br>U 2.0<br>99.9 | ||
| + | | bgcolor="#FFFFCC" | <br>U 1.009<br>L 1.328 | ||
|- | |- | ||
| − | | | + | | colspan="5" align="center" | '''with various [[ester]]s''' |
|- | |- | ||
| − | | | + | | [[ethyl acetate]] || 77.1 || 70.4 || 91.9<br>U 96.7<br>L 8.7 |
| + | | bgcolor="#FFFFCC" | <br>U 0.907<br>L 0.999 | ||
| + | |- bgcolor="#FFFFCC" | ||
| + | | [[methyl acetate]] || 57.0 || 56.1 || 95.0 || 0.940 | ||
| + | |- bgcolor="CCFFFF" | ||
| + | | [[n-propyl acetate]]<ref name="metalfinishing">{{Cite web | ||
| + | | url = http://www.metalfinishing.com/_virtual/article-downloads/Table%201_a2648.pdf | ||
| + | | title = Binary Organic Azeotropes Useful for Solvent Cleaning | ||
| + | | accessdate = 13 February 2011 | ||
| + | | author = John Durkee | ||
| + | | last = | ||
| + | | first = | ||
| + | | authorlink = | ||
| + | | coauthors = | ||
| + | | date = | ||
| + | | year = 2000 | ||
| + | | month = November | ||
| + | | format = | ||
| + | | work = | ||
| + | | publisher = metalfinishing.com | ||
| + | | pages = | ||
| + | | language = | ||
| + | | archiveurl = | ||
| + | | archivedate = | ||
| + | | quote = | ||
| + | }} </ref> || 101.6 || 82.4 || 86 || | ||
| + | |- bgcolor="FFCCFF" | ||
| + | | [[ethyl nitrate]] || 87.7 || 74.4 || 78 || | ||
|- | |- | ||
| − | | | + | | colspan="5" align="center" | '''with various other [[solvent]]s''' |
| + | |- bgcolor="#CCFFFF" | ||
| + | | [[acetone]] <sup>‡</sup><ref name="br"/><ref name="hilmen"/> || 56.5 °C | ||
| + | | colspan="3" align="center" | No azeotrope | ||
|- | |- | ||
| − | | | + | | [[methyl ethyl ketone]] || 79.6 || 73.5 || 89 |
| + | | bgcolor="#FFFFCC" | 0.834 | ||
|- | |- | ||
| − | | | + | | [[pyridine]] || 115.5 || 92.6 || 57 |
| + | | bgcolor="#FFFFCC" | 1.010 | ||
|- | |- | ||
| − | | | + | | [[benzene]] || 80.2 || 69.3 || 91.1<br>U 99.94<br>L 0.07 |
| + | | bgcolor="#FFFFCC" | <br>U 0.880<br>L 0.999 | ||
|- | |- | ||
| − | | | + | | [[toluene]] || 110.8 || 84.1 || 79.8<br>U 99.95<br>L 0.06 |
| + | | bgcolor="#FFFFCC" | <br>U 0.868<br>L 1.000 | ||
| + | |- bgcolor="#FFFFCC" | ||
| + | | [[cyclohexane]] || 80.7 || 69.8 || 91.5<br>U 99.99<br>L 0.01||<br>U 0.780<br>L 1.00 | ||
|- | |- | ||
| + | | [[diethyl ether]] || 34.5 || 34.2 || 98.7 | ||
| + | | bgcolor="#FFFFCC" | 0.720 | ||
| + | |- bgcolor="#CCFFFF" | ||
| + | | [[tetrahydrofuran]]<ref name="br"/> || 66 || 65 || 95 || | ||
| + | |- bgcolor="#FFCCFF" | ||
| + | | [[anisole]] || 153.9 || 95.5 || 59.5 || | ||
| + | |- bgcolor="#FFFFCC" | ||
| + | | [[acetonitrile]] || 82.0 || 76.5 || 83.7 | ||
| + | | bgcolor="#FFFFCC" | 0.818 | ||
| + | |- bgcolor="#FFFFCC" | ||
| + | | [[chloral]] || 97.75 || 95.0 || 93.0 || | ||
| + | |- bgcolor="#CCFFFF" | ||
| + | | [[hydrazine]]<ref name="merck4653">''Merck Index of Chemicals and Drugs, 9th ed.'' monograph 4653</ref> || 113.5 °C || 120.3 °C || 68.5 || | ||
|} | |} | ||
Latest revision as of 00:38, 9 February 2013
page in progress....
Contents
Solvents
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| Acetone | 56 °C | Miscible | 0.786 g/ml | 58.08 g/mol |
Note about Density: If the density of the solvent is greater then one (mass > 1.00) the solvent, if non-polar will sink below water.
likewise if the density is less then one (mass < 1.00) the solvent, if non-polar, will float on the surface of the water
Polar protic
Water
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| Water | 100 °C | Miscible | 1.000 g/ml | 18.02 g/mol |
Carboxylic acids
Formic acid
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| Formic acid | 100.8 °C | Miscible | 1.22 g/ml | 46.03 g/mol |
Acetic acid
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| Acetic acid | 118 °C | Miscible | 1.049 g/ml | 60.05 g/mol |
Alcohols
Ethanol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C2H5OH | 79 °C | Miscible | 0.789 g/ml | 46.07 g/mol |
Azeotrope % weight /w water = 95.5%
Miscible in:
Methanol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C2H5OH | 65 °C | Miscible | 0.791 g/ml | 32.04 g/mol |
No azeotrope w/ water
Miscible in:
Propanol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C2H5OH | 97 °C | Miscible | 0.803 g/ml | 60.1 g/mol |
Azeotrope % weight /w water = 71.7%
Miscible in:
Isopropanol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C3H7OH | 82.5 °C | Miscible | 0.786 g/ml | 60.1 g/mol |
Azeotrope % weight /w water = 87.9%
Miscible in: non-polar solvents, haloforms, water
Glycerol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C3H5(OH)3 | 290 °C | Miscible | 1.261 g/ml | 92.09 g/mol |
Azeotrope % weight /w water =
Miscible in: water, alcohols
Propylene Glycol
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C3H7OH | 188.2 °C | Miscible | 1.036 g/ml | 76.09 g/mol |
Azeotrope % weight /w water =
Miscible in:
Polar aprotic
Ketones
Short chain ketones are miscible in ALL organic solvents
Acetone
| Formula | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C3H6O | 56 °C | Miscible | 0.786 g/ml | 58.08 g/mol |
Miscible in:
All organic solvents
- Does not from an Azeotrope with water
Methyl ethyl ketone (MEK)
| Formula | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C4H8O | 80 °C | Miscible 275. g/L | 0.805 g/ml | 72.11 g/mol |
Miscible in:
All organic solvents
Cyclohexanone
| Formula | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C6H10O | 155.65 °C | Miscible 90 g/L | 0.9478 g/ml | 98.15 g/mol |
Miscible in:
All organic solvents
Esters
Ethyl acetate
Ethers
Tetrahydrofuran
Others
Dimethyl sulfoxide (DMSO)
Acetonitrile
Dimethylformamide
Non-polar
Aliphatic
Butane
Pentane
Hexane
Heptane
Octane
Cyclic
Cyclohexane
Cyclopentane
Aromatic
Benzene
Toluene
Xylene
Ethers
Diethyl ether
Chlorinated hydrocarbons (only slighlty polar)
Chloroform
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C2H5OH | 61.2 °C | Immiscible | 1.483 g/ml | 199.38 g/mol |
Azeotrope % weight /w water = 97.0%
Miscible in: non-polar solvents, alcohols
Carbon Tetrachloride
Dichloromethane (DCM)
| Solvent | Boiling Point °C | Solubility /w Water | Density | Molar mass | |
|---|---|---|---|---|---|
| C2H5OH | 39.6 °C | Immiscible | 1.33 g/ml | 84.93 g/mol |
Azeotrope % weight /w water = 98.5%
Azeotrope % weight /w methanol = 7.3%
Azeotrope % weight /w diethyl ether = 30%
Miscible in: non-polar solvents, alcohols
Other
d-limonene
Common Azeotropes
(taken from wikipedia)
Azeotropes of water, b.p.=100 °C
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cite error: <ref> tags exist, but no <references/> tag was found