Difference between revisions of "Hordenine"
From DMT-Nexus Wiki
TheTraveler (Talk | contribs) (Created page with "Category:Alkaloids") |
(→Natural sources of hordenine) |
||
| (28 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Alkaloids]] | [[Category:Alkaloids]] | ||
| + | |||
| + | [[Image:hordeninefreebase.png|right]] | ||
| + | |||
| + | == What is hordenine == | ||
| + | |||
| + | * ''Hordenine'' (N,N-dimethyl-4-hydroxyphenylethylamine) is a phenethylamine alkaloid present in a range of plant species, with unknown human activity. It is suspected to have stimulant or 'ephedra like' effects. | ||
| + | |||
| + | == Chemical and physical properties == | ||
| + | |||
| + | [Hordenine properties http://wiki.dmt-nexus.me/Psychedelic_Compounds_Chemical_and_Physical_Properties#Hordenine] | ||
| + | |||
| + | == Biochemistry and pharmacology == | ||
| + | |||
| + | * Radiolabeling showed that tyrosine was the biosynthetic precursor to both HMePEA and hordenine in barley (Leete and Marion, 1953). | ||
| + | |||
| + | * Action on isolated frog heart was studied (Ellis, 1949). LD50 for mice determined (Aliev et, 1967). Effects on the Heobania vermiculata nerve cell firing studied (Avoli et al., 1978). | ||
| + | |||
| + | * Tyramine (HPEA) and N-methyltyramine (HMePEA) were equally effective as pressors and bronchodilators (at 1 ug/ kg). Hordenine is only one tenth as potent, but the quaternary salt HM3 PEA) was more potent than all three (Alles, 19~3). Hordenine has sympathacolytic actions; large doses decreases or inverts the hypertenstve actwn of adrenaline (Raymond Hamet, 1936). | ||
| + | |||
| + | *.Enzymatic oxidative deamination and behavioral effects on the cat were observed (Clark et al., 1964); hordenine was also studied in relationship to the compulsive gnaw syndrome in the rat {Ernst, 1965b). Hordenine, in i.v. and oral administration in sheep, induced the clinical signs of Phalaris toxicity but not the cardiac sudden death syndrome (Bourke et al.,1988, 2006). | ||
| + | |||
| + | * Hordenine was studied in laboratory animals, along with 32 other ring-substituted phenethylamines, as protective agents against ionizing radiation (ll'yuchenok et al., 1976). The occasional observation of hordenine in the urine of racehorses led to a pharmacological study of hordenine administration for performance enhancement (Frank et al., 1990; Hapke and Strathman, 1995). | ||
| + | |||
| + | * Human psychoactivity of hordenine is unknown. | ||
| + | |||
| + | == Natural sources of hordenine == | ||
| + | |||
| + | === Hordeum spp === | ||
| + | |||
| + | * Un-sprouted barley contains no hordenine, but it appears following germination. During the four days immediately after germination, the hordenine content was highest, and decreased as the embryo grew older, so that after 25 days no hordenine was present (Torquati 1911). Both hordenine and gramine are biosynthesized in barley malt during germination (Mangino and Scanlan, 1984). Ten grams of fresh barley rootlets upon extraction with dilute sulfuric acid and clean-up, yielded 1.2 mg of pure hordenine (Raout 1934). The major source of hordenine consumption in humans is from beer brewed from barley (Singh et al, 1992). ref Shulgin Index | ||
| + | |||
| + | |||
| + | === Acacia spp. === | ||
| + | |||
| + | * Extraction of NH40H moistened leaves and twigs of Acacia harpophylla, and similarly heated tissues of A. holosericea, A kettlewelliae, A. adunca, and A harpophylla produced hor denine (Fitzgerald, 1964). Hordenine was reported in A. berlandieri (Clement et aL, 1997) A rigidula (Clement et aL, 1998), and in fresh trunk bark from A spirorlis (Poupat and Sevenet 1975). Hordenine, tyramine, and HMePEA were isolated from Acacia berlandieri (Adams and Camp, 1966). | ||
| + | |||
| + | === Aconitum spp. === | ||
| + | * Hordenine in Aconitum tanguticum (Wang et al., 2002), | ||
| + | |||
| + | === Algae === | ||
| + | |||
| + | * Hordenine hns been also observed in the marine algae Phyllophora nervosa (Guvcn et al., 1969, 1970), Ahnfeltia paradoxa (Kawauchi and Sasaki, 1978), and the marine red alga Gigartina stellata (Barwell and Blunden, 1981). | ||
| + | |||
| + | === Alhagi spp. === | ||
| + | * hordenine found in the stem (and to a lesser extent, the root) of Alhagi pseudalhagi (Ghosal and Srivastava, 1973b) | ||
| + | |||
| + | === Ariocarpus spp. === | ||
| + | |||
| + | * Ariocarpus kotschoubeyanus (Neal et al., 197la), A fissuratus, vars. fissuratus and lloydii (McLaughlin, 1969) A retusus (Braga and McLaughlin, 1969), A trigonus (Speir et aL 1970) | ||
| + | |||
| + | === Cannabis spp. === | ||
| + | * Traces of Hordenine was identified in the dried leaves of Cannabis sativa (El-Feraly and Turner 1975). | ||
| + | |||
| + | === Desmodium === | ||
| + | |||
| + | * Hordenine has been found in the legumes Desmodium gangeticum (Ghosal and Bhattacharaya, 1972), D. tiliaefolium (Ghosal and Srivastava, 1973a), in Desmodium triflorum (Ghosal et al., 1973) and D. floribundu G. Don. (Maurya et al., 1985) | ||
| + | |||
| + | === Echinocereus spp === | ||
| + | |||
| + | * Echinocerus candicans, previously classified as [[Trichocereus candicans]], Hordenine found to be 0.5-5.0% of alkaloid (Reti, L. 1953). | ||
| + | |||
| + | * Echinocereus merkeri, hordenine also found to be present (Agurell et al 1969). | ||
| + | |||
| + | === Ephedra spp. === | ||
| + | |||
| + | * Ephedra aphylla (Abdel-Kader et al., 2003). | ||
| + | |||
| + | === Haloxylon spp. === | ||
| + | * Haloxylon salicomicum (El-Shazly et al., 2005). | ||
| + | |||
| + | === Lophophora spp. === | ||
| + | |||
| + | [[Lophophora williamsii]] | ||
| + | * An alkaloid (anhaline) from Lophophora williamsii was shown to be hordenine (Spath, 1919) and further proven to be identical with hordenine by th.e comparison of the mixed melting points of the picrate, the perchlorate, the methiodide, and the 0 -acetyl ester hydrochloride (Spath, 1921). The L. illiamsii alkaloid peyocactin was shown to be hordenine (Rao, 1970). It has also been found in L. williamsii by others (Lundstrom and Agurell, 1968a). | ||
| + | * Hordenine, tyramine, and HMePEA were isolated from Lophophora williamsii (McLaughlin and Paul, 1966). | ||
| + | |||
| + | === Pancratium spp. === | ||
| + | * Hordenine has been isolated from Pancratium maritimum (Sandberg et al. 1963) | ||
| + | |||
| + | === Panicum spp === | ||
| + | |||
| + | * The accumulation of hordenine in the seedlings of Panicum miliaceum continues for at least eight days (Demaree and Tyler, 1956). | ||
| + | |||
| + | === Phalaris spp. === | ||
| + | |||
| + | [[Phalaris arundinacea]] | ||
| + | * Reed canary grass contains both hordenine (Audette et al., 1969) and several indolealkylamines; meadow voles were used to estimate the toxicity of this grass as forage material (Goelz et al., 1980). Quantitative analyses were made of P. arundinacea (Duynisveld et al., 1990; Kalen et al., 1992). Hordenine and 5-MeO-NMT (5-methoxy-N-methyltryptamine) were also isolated from P. arundinaceae (Wilkinson, 1958; Bourke et al., 1988). | ||
| + | |||
| + | [[Phalaris aquatica]] | ||
| + | * The acute toxicity of Phalaris aquatica (also known as P. tuberosa, Harding grass) is purported to be due to three alkaloids that are present: gramine, 5-MeO-DMT, and hordenine (Bourke et al., 1988), though other phenethylamines have also be implicated (Culvenor el al. 2005; Bourke et al., 2008). | ||
| + | |||
| + | === Sageretia === | ||
| + | |||
| + | * from shrubs of the Rhamnaceae: Sageretia hamosa, S. he11ryi, S. melliana, and S. thea (Zhong et al., 1994), | ||
| + | |||
| + | === Securinega spp. === | ||
| + | * Hordenine isolated from the roots of Securinega virosa (Iketubosin and Mathieson, 1963). . | ||
| + | |||
| + | === Selenicereus spp. === | ||
| + | * Hordenine ;vas isolated from Selenicereus grandiflorus and S. pterantltus (Petershofer-Halbmayer et al., 1982), | ||
| + | |||
| + | |||
| + | === Other cactus === | ||
| + | |||
| + | * Hordenine present in Gymnocalycium saglione (Nieto et aL, 1982), Gymnocalycium spp. (Starha, 1996), Gymnocalycium schickendantzii and Cereus aethiops (Ruiz et aL, 1973) | ||
| + | * Mammillaria microcarpa (Howe et aL, 1977a) | ||
| + | * Obregonia deneg1'ii (Neal et al 1971b), | ||
| + | * Islaya minor Backbg. (Doetsch et aL, 1980) | ||
| + | * Ariocarpus agavoides and Pelecyphora aselliformis (Bruhn and Bruhn, 1973; Neal et aL 1972), | ||
| + | * Coryphantha rarnillosa (Sato et aL, 1973), C macromeris var. runyonii (Keller et aL, 1973) and C (Neobesseya) missouriensis (Pummangura et aL, 1981), seven species of Coryphanta. (Homemann et aL, 1972). Hordenine was found in Coryphanta greenwoodii, C bumamma, and three additional species (Bruhn et aL, 1975) and was the sole alkaloid found in Colyphantha vivipara var arizonica (Howe et aL, 1977b) | ||
| + | * seven species of the cactus genus Gymnocactus specifically G. aguirreanus, G. beguinii, G. horripilus, G. knuthianus, G. mandragora, G. roseanus, and G. vierechi (West et aL, 1974), | ||
| + | * Ariocarpus scapharostrus (Bruhn, 1975), | ||
| + | * Lobivia backebergii, L bingharniana, L pentlandii, and Pseudolol1ivia kermesina (Follas et aL, 1977), | ||
| + | * Espostoa huanucensis (Mata et aL, 1976) | ||
| + | * Dolichothele surculosa and other Dolichothele species (Dingerdissen and McLaughlin, 1973), | ||
| + | * Turbinicarpus spedes (Starha et aL, 1999) | ||
| + | * the Opuntia subgenera Cylindropuntia and other Opuntia spp. (Meyer et aL, 1980). . Hordenine, along with tyramine, 3-methoxytyramine, and N-methyltyramine were found in Opuntia species (Meyer et aL, 1980). | ||
| + | * Hordenine was isolated from Stapelia gigantea (Keller, 1981) | ||
| + | * hordenine, its glucoside and its acetate ester, the quaternary amine candicine, and the flavone luteolin were isolated from the African cactus Stapelia hirsuta L, commonly known as window sill cactus (Shabana et aL, 2006). | ||
| + | |||
| + | == Analysis == | ||
| + | |||
| + | === GC-MS EI === | ||
| + | |||
| + | http://webbook.nist.gov/cgi/cbook.cgi?ID=C539151&Mask=200#Mass-Spec | ||
| + | |||
| + | === LC-MS ESI === | ||
| + | |||
| + | http://131.113.122.76/jsp/Dispatcher.jsp?type=disp&id=WA002706&site=2 | ||
Latest revision as of 16:49, 29 May 2024
Contents
- 1 What is hordenine
- 2 Chemical and physical properties
- 3 Biochemistry and pharmacology
- 4 Natural sources of hordenine
- 4.1 Hordeum spp
- 4.2 Acacia spp.
- 4.3 Aconitum spp.
- 4.4 Algae
- 4.5 Alhagi spp.
- 4.6 Ariocarpus spp.
- 4.7 Cannabis spp.
- 4.8 Desmodium
- 4.9 Echinocereus spp
- 4.10 Ephedra spp.
- 4.11 Haloxylon spp.
- 4.12 Lophophora spp.
- 4.13 Pancratium spp.
- 4.14 Panicum spp
- 4.15 Phalaris spp.
- 4.16 Sageretia
- 4.17 Securinega spp.
- 4.18 Selenicereus spp.
- 4.19 Other cactus
- 5 Analysis
What is hordenine
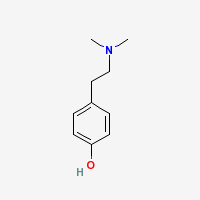
- Hordenine (N,N-dimethyl-4-hydroxyphenylethylamine) is a phenethylamine alkaloid present in a range of plant species, with unknown human activity. It is suspected to have stimulant or 'ephedra like' effects.
Chemical and physical properties
[Hordenine properties http://wiki.dmt-nexus.me/Psychedelic_Compounds_Chemical_and_Physical_Properties#Hordenine]
Biochemistry and pharmacology
- Radiolabeling showed that tyrosine was the biosynthetic precursor to both HMePEA and hordenine in barley (Leete and Marion, 1953).
- Action on isolated frog heart was studied (Ellis, 1949). LD50 for mice determined (Aliev et, 1967). Effects on the Heobania vermiculata nerve cell firing studied (Avoli et al., 1978).
- Tyramine (HPEA) and N-methyltyramine (HMePEA) were equally effective as pressors and bronchodilators (at 1 ug/ kg). Hordenine is only one tenth as potent, but the quaternary salt HM3 PEA) was more potent than all three (Alles, 19~3). Hordenine has sympathacolytic actions; large doses decreases or inverts the hypertenstve actwn of adrenaline (Raymond Hamet, 1936).
- .Enzymatic oxidative deamination and behavioral effects on the cat were observed (Clark et al., 1964); hordenine was also studied in relationship to the compulsive gnaw syndrome in the rat {Ernst, 1965b). Hordenine, in i.v. and oral administration in sheep, induced the clinical signs of Phalaris toxicity but not the cardiac sudden death syndrome (Bourke et al.,1988, 2006).
- Hordenine was studied in laboratory animals, along with 32 other ring-substituted phenethylamines, as protective agents against ionizing radiation (ll'yuchenok et al., 1976). The occasional observation of hordenine in the urine of racehorses led to a pharmacological study of hordenine administration for performance enhancement (Frank et al., 1990; Hapke and Strathman, 1995).
- Human psychoactivity of hordenine is unknown.
Natural sources of hordenine
Hordeum spp
- Un-sprouted barley contains no hordenine, but it appears following germination. During the four days immediately after germination, the hordenine content was highest, and decreased as the embryo grew older, so that after 25 days no hordenine was present (Torquati 1911). Both hordenine and gramine are biosynthesized in barley malt during germination (Mangino and Scanlan, 1984). Ten grams of fresh barley rootlets upon extraction with dilute sulfuric acid and clean-up, yielded 1.2 mg of pure hordenine (Raout 1934). The major source of hordenine consumption in humans is from beer brewed from barley (Singh et al, 1992). ref Shulgin Index
Acacia spp.
- Extraction of NH40H moistened leaves and twigs of Acacia harpophylla, and similarly heated tissues of A. holosericea, A kettlewelliae, A. adunca, and A harpophylla produced hor denine (Fitzgerald, 1964). Hordenine was reported in A. berlandieri (Clement et aL, 1997) A rigidula (Clement et aL, 1998), and in fresh trunk bark from A spirorlis (Poupat and Sevenet 1975). Hordenine, tyramine, and HMePEA were isolated from Acacia berlandieri (Adams and Camp, 1966).
Aconitum spp.
- Hordenine in Aconitum tanguticum (Wang et al., 2002),
Algae
- Hordenine hns been also observed in the marine algae Phyllophora nervosa (Guvcn et al., 1969, 1970), Ahnfeltia paradoxa (Kawauchi and Sasaki, 1978), and the marine red alga Gigartina stellata (Barwell and Blunden, 1981).
Alhagi spp.
- hordenine found in the stem (and to a lesser extent, the root) of Alhagi pseudalhagi (Ghosal and Srivastava, 1973b)
Ariocarpus spp.
- Ariocarpus kotschoubeyanus (Neal et al., 197la), A fissuratus, vars. fissuratus and lloydii (McLaughlin, 1969) A retusus (Braga and McLaughlin, 1969), A trigonus (Speir et aL 1970)
Cannabis spp.
- Traces of Hordenine was identified in the dried leaves of Cannabis sativa (El-Feraly and Turner 1975).
Desmodium
- Hordenine has been found in the legumes Desmodium gangeticum (Ghosal and Bhattacharaya, 1972), D. tiliaefolium (Ghosal and Srivastava, 1973a), in Desmodium triflorum (Ghosal et al., 1973) and D. floribundu G. Don. (Maurya et al., 1985)
Echinocereus spp
- Echinocerus candicans, previously classified as Trichocereus candicans, Hordenine found to be 0.5-5.0% of alkaloid (Reti, L. 1953).
- Echinocereus merkeri, hordenine also found to be present (Agurell et al 1969).
Ephedra spp.
- Ephedra aphylla (Abdel-Kader et al., 2003).
Haloxylon spp.
- Haloxylon salicomicum (El-Shazly et al., 2005).
Lophophora spp.
- An alkaloid (anhaline) from Lophophora williamsii was shown to be hordenine (Spath, 1919) and further proven to be identical with hordenine by th.e comparison of the mixed melting points of the picrate, the perchlorate, the methiodide, and the 0 -acetyl ester hydrochloride (Spath, 1921). The L. illiamsii alkaloid peyocactin was shown to be hordenine (Rao, 1970). It has also been found in L. williamsii by others (Lundstrom and Agurell, 1968a).
- Hordenine, tyramine, and HMePEA were isolated from Lophophora williamsii (McLaughlin and Paul, 1966).
Pancratium spp.
- Hordenine has been isolated from Pancratium maritimum (Sandberg et al. 1963)
Panicum spp
- The accumulation of hordenine in the seedlings of Panicum miliaceum continues for at least eight days (Demaree and Tyler, 1956).
Phalaris spp.
- Reed canary grass contains both hordenine (Audette et al., 1969) and several indolealkylamines; meadow voles were used to estimate the toxicity of this grass as forage material (Goelz et al., 1980). Quantitative analyses were made of P. arundinacea (Duynisveld et al., 1990; Kalen et al., 1992). Hordenine and 5-MeO-NMT (5-methoxy-N-methyltryptamine) were also isolated from P. arundinaceae (Wilkinson, 1958; Bourke et al., 1988).
- The acute toxicity of Phalaris aquatica (also known as P. tuberosa, Harding grass) is purported to be due to three alkaloids that are present: gramine, 5-MeO-DMT, and hordenine (Bourke et al., 1988), though other phenethylamines have also be implicated (Culvenor el al. 2005; Bourke et al., 2008).
Sageretia
- from shrubs of the Rhamnaceae: Sageretia hamosa, S. he11ryi, S. melliana, and S. thea (Zhong et al., 1994),
Securinega spp.
- Hordenine isolated from the roots of Securinega virosa (Iketubosin and Mathieson, 1963). .
Selenicereus spp.
- Hordenine ;vas isolated from Selenicereus grandiflorus and S. pterantltus (Petershofer-Halbmayer et al., 1982),
Other cactus
- Hordenine present in Gymnocalycium saglione (Nieto et aL, 1982), Gymnocalycium spp. (Starha, 1996), Gymnocalycium schickendantzii and Cereus aethiops (Ruiz et aL, 1973)
- Mammillaria microcarpa (Howe et aL, 1977a)
- Obregonia deneg1'ii (Neal et al 1971b),
- Islaya minor Backbg. (Doetsch et aL, 1980)
- Ariocarpus agavoides and Pelecyphora aselliformis (Bruhn and Bruhn, 1973; Neal et aL 1972),
- Coryphantha rarnillosa (Sato et aL, 1973), C macromeris var. runyonii (Keller et aL, 1973) and C (Neobesseya) missouriensis (Pummangura et aL, 1981), seven species of Coryphanta. (Homemann et aL, 1972). Hordenine was found in Coryphanta greenwoodii, C bumamma, and three additional species (Bruhn et aL, 1975) and was the sole alkaloid found in Colyphantha vivipara var arizonica (Howe et aL, 1977b)
- seven species of the cactus genus Gymnocactus specifically G. aguirreanus, G. beguinii, G. horripilus, G. knuthianus, G. mandragora, G. roseanus, and G. vierechi (West et aL, 1974),
- Ariocarpus scapharostrus (Bruhn, 1975),
- Lobivia backebergii, L bingharniana, L pentlandii, and Pseudolol1ivia kermesina (Follas et aL, 1977),
- Espostoa huanucensis (Mata et aL, 1976)
- Dolichothele surculosa and other Dolichothele species (Dingerdissen and McLaughlin, 1973),
- Turbinicarpus spedes (Starha et aL, 1999)
- the Opuntia subgenera Cylindropuntia and other Opuntia spp. (Meyer et aL, 1980). . Hordenine, along with tyramine, 3-methoxytyramine, and N-methyltyramine were found in Opuntia species (Meyer et aL, 1980).
- Hordenine was isolated from Stapelia gigantea (Keller, 1981)
- hordenine, its glucoside and its acetate ester, the quaternary amine candicine, and the flavone luteolin were isolated from the African cactus Stapelia hirsuta L, commonly known as window sill cactus (Shabana et aL, 2006).
Analysis
GC-MS EI
http://webbook.nist.gov/cgi/cbook.cgi?ID=C539151&Mask=200#Mass-Spec
LC-MS ESI
http://131.113.122.76/jsp/Dispatcher.jsp?type=disp&id=WA002706&site=2